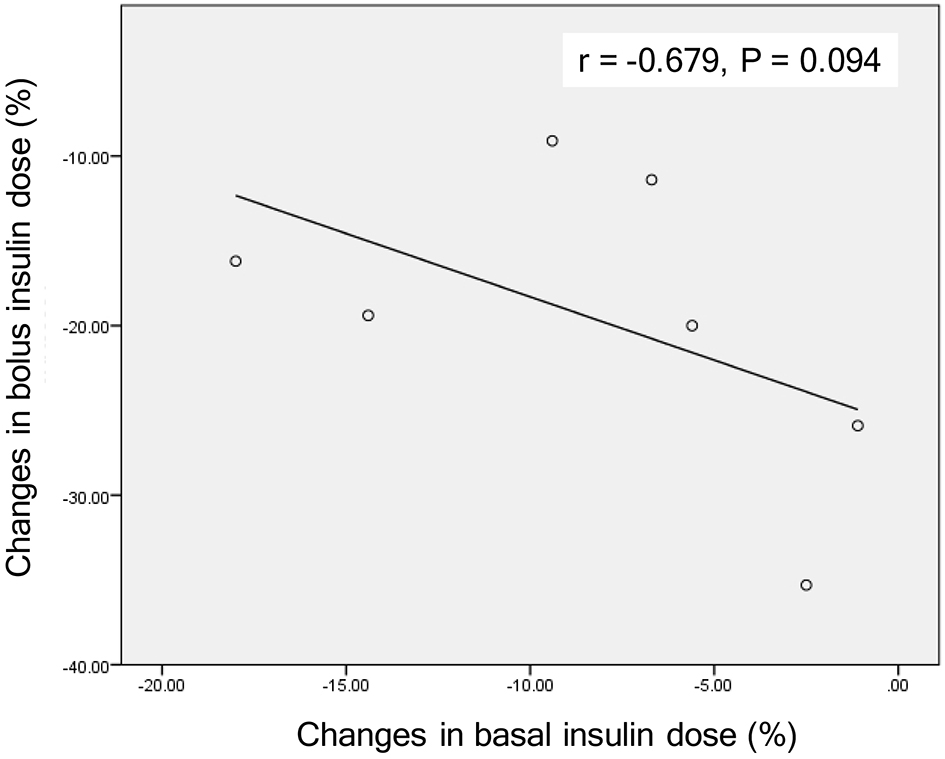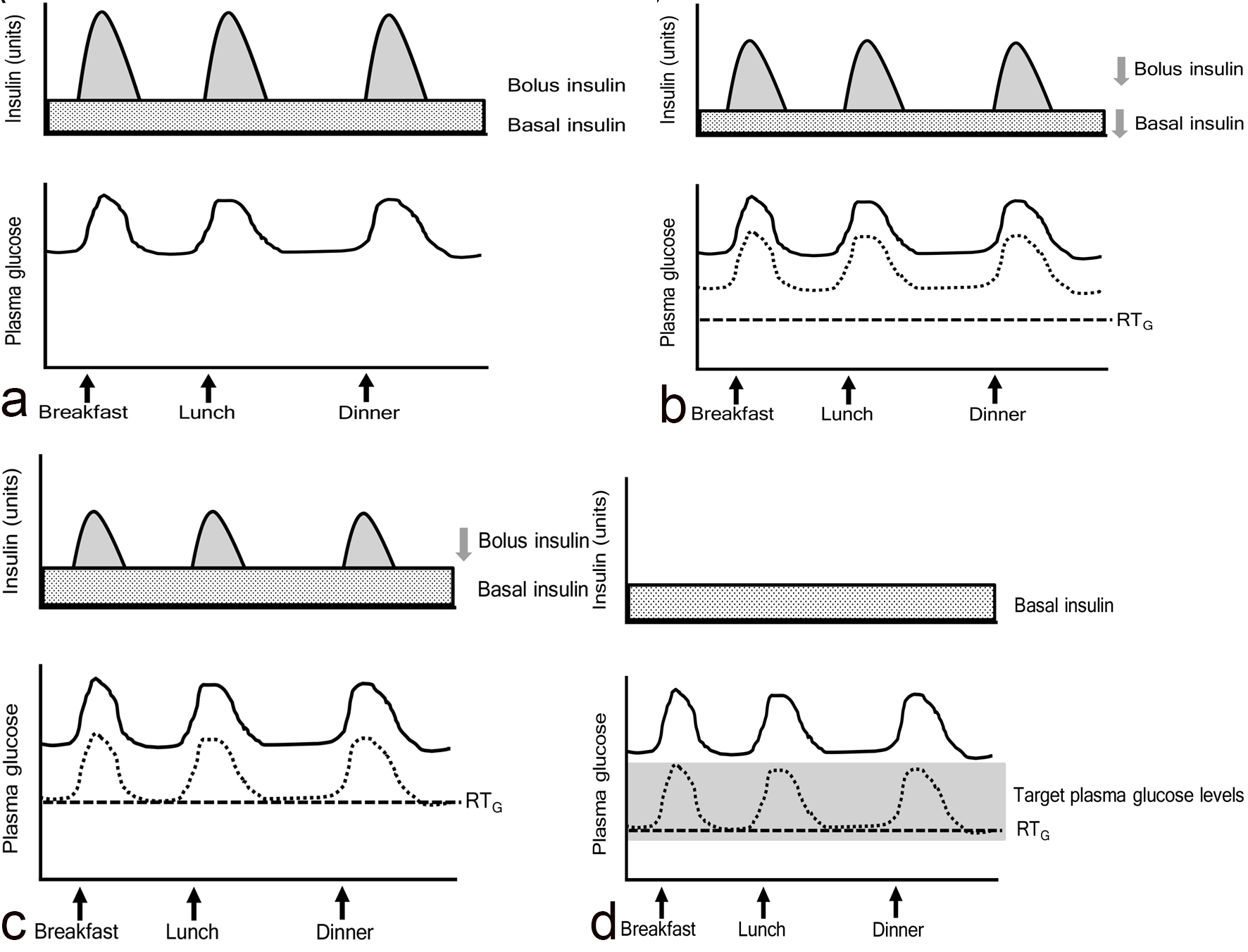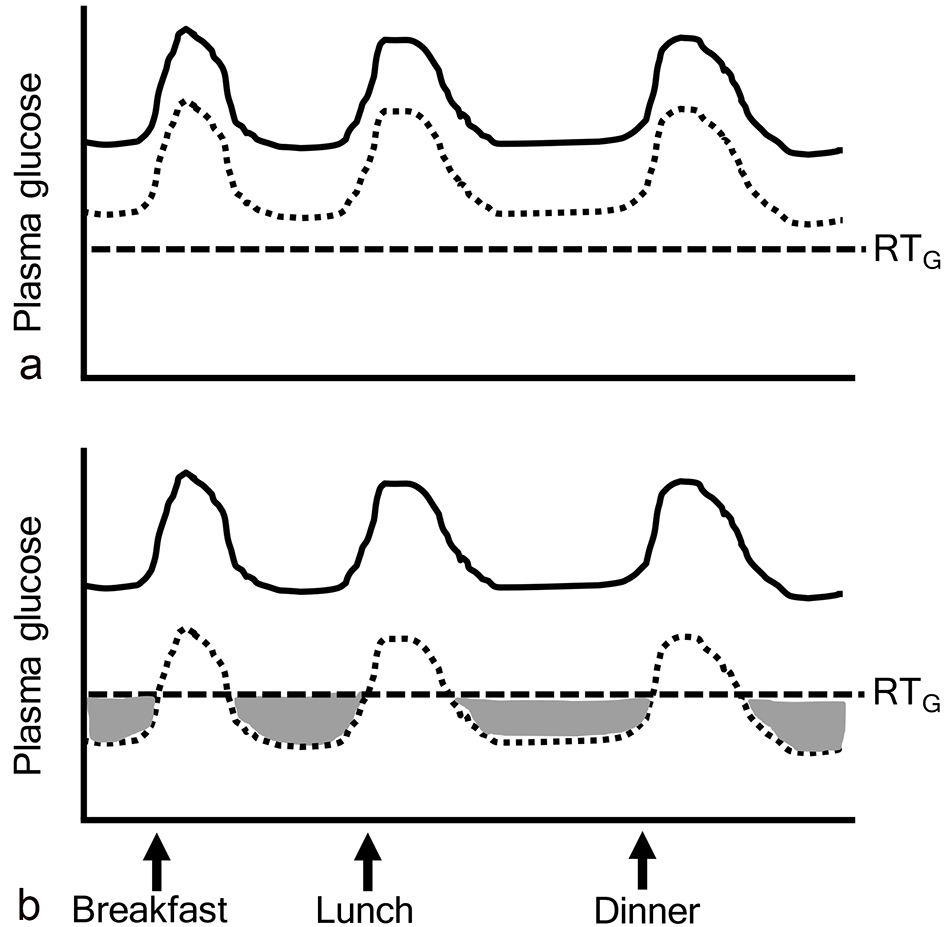
Figure 1. Ineffective range of SGLT2 inhibitors. The gray area indicates “ineffective range of SGLT2i” which shows that plasma glucose levels are below the renal threshold for glucose excretion (RTG). Solid and dotted lines indicate plasma glucose levels before and after the addition of SGLT2 inhibitors, respectively.