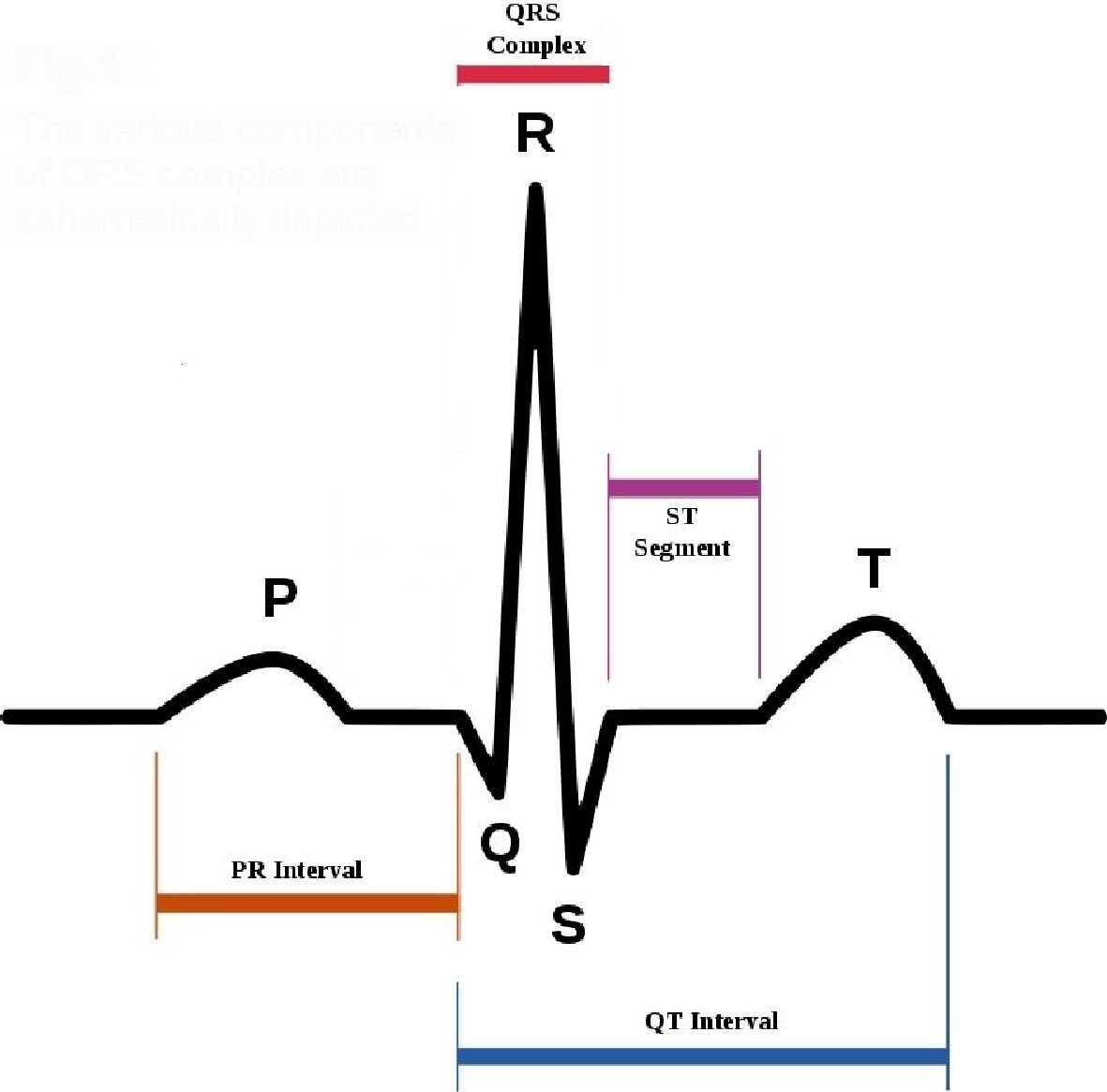
Figure 1. The various components of QRS complex are schematically depicted.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 7, July 2018, pages 593-600
Malignant Ventricular Arrhythmias Resulting From Drug-Induced QTc Prolongation: A Retrospective Study
Figures

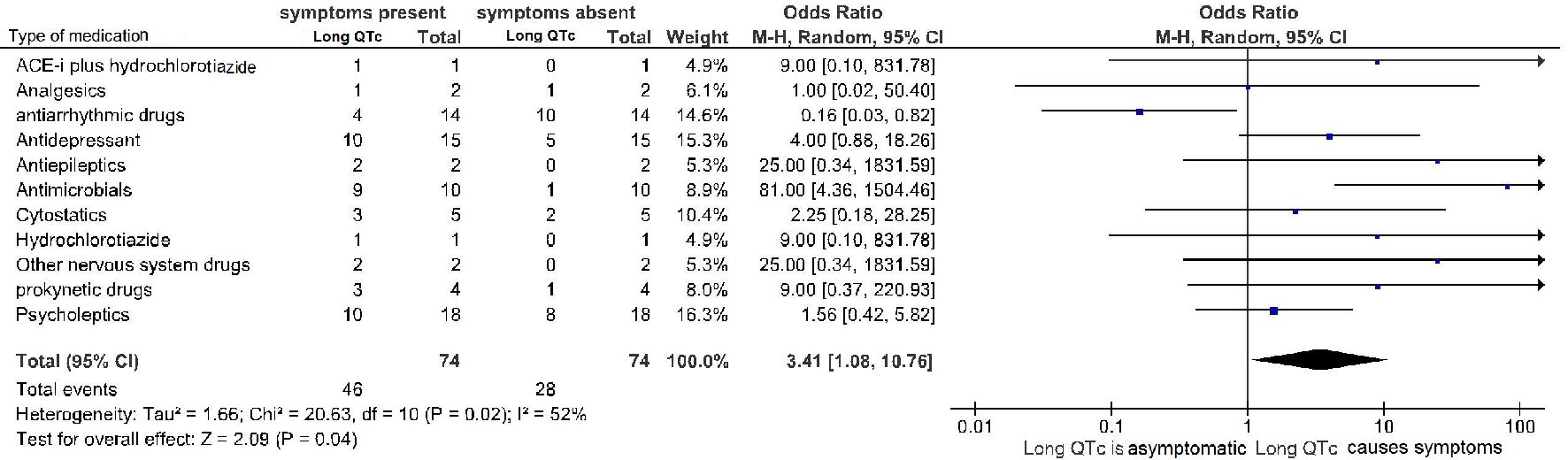
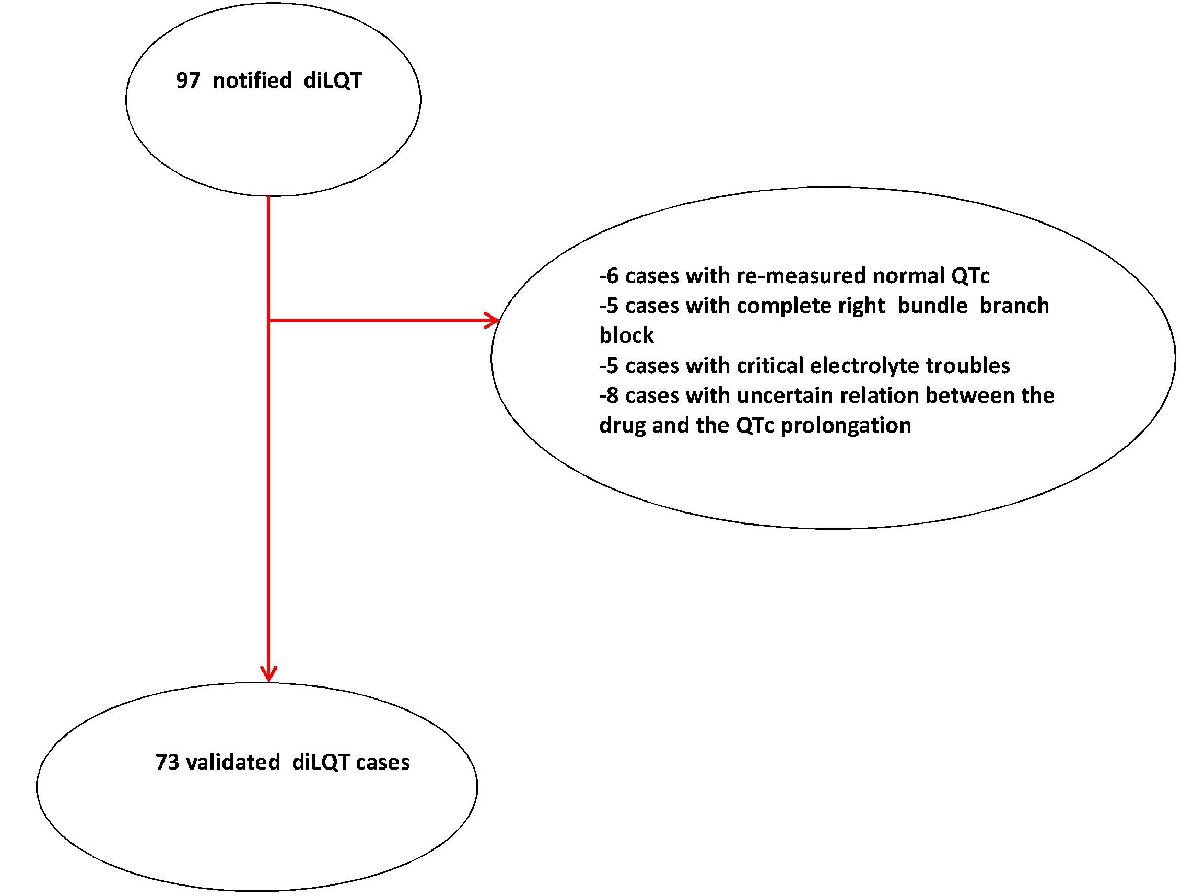
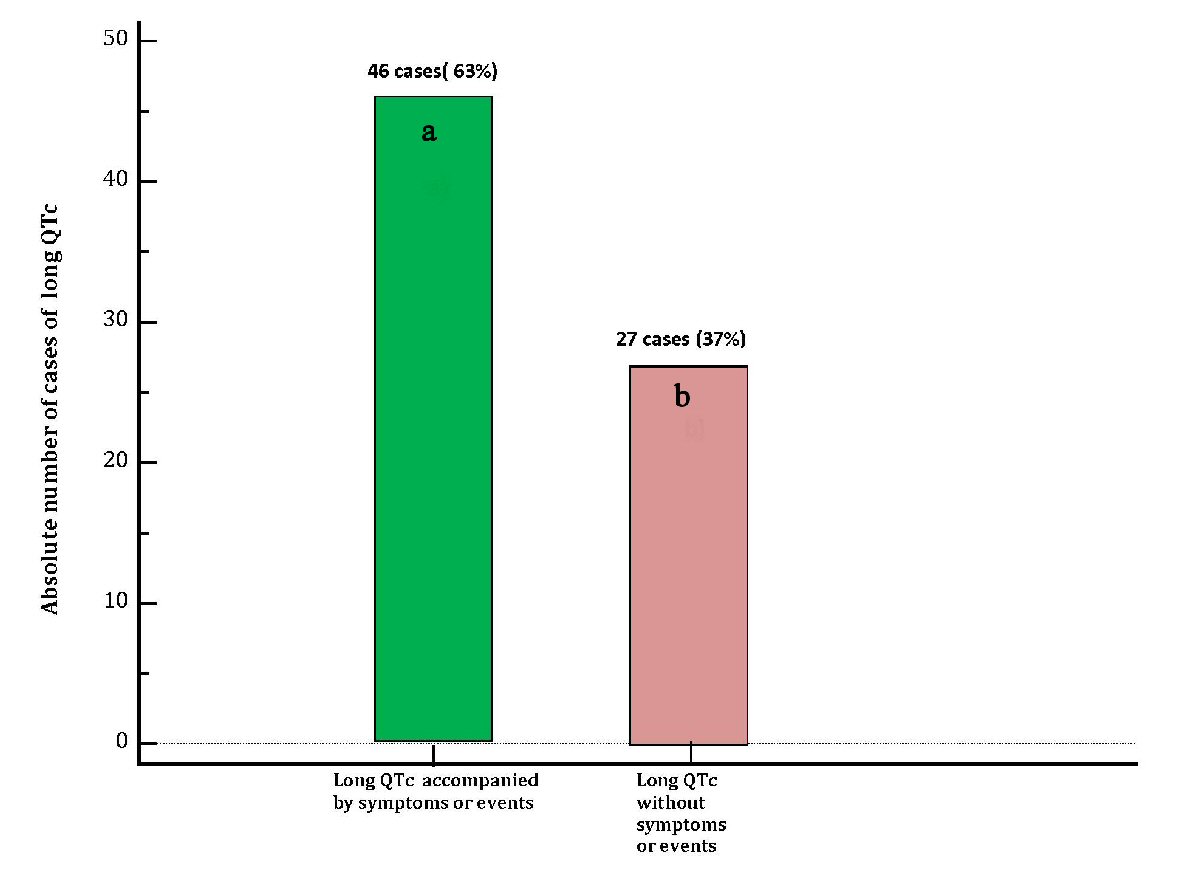
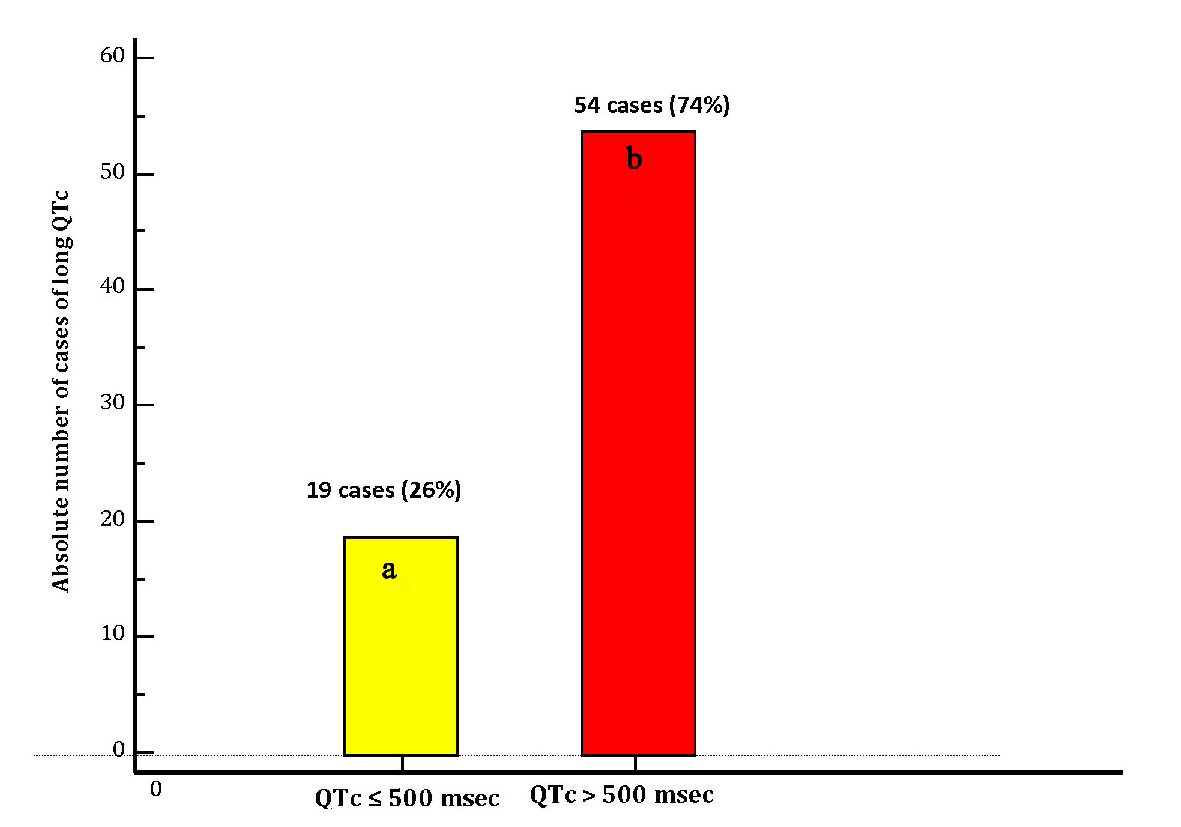
Tables
| DiLQTc patients with symptoms (dizziness, near-fainting, syncope) or events(TdP, VF) (n = 46) | DiLQTc patients without symptoms or events (n = 27) | P-value | |
|---|---|---|---|
| diLQTc: drug-induced long heart rate-corrected QT interval; TdP: torsade de pointes; VF: ventricular fibrillation; N/A: not applicable. | |||
| Age( mean ± SD), years | 58 ± 10.5 | 62 ± 8.5 | 0.0972 |
| Male sex, number (%) | 23 (50 %) | 13 (48% ) | 0.9285 |
| Severe QTc ( > 500 ms) | 34 (73.9 % ) | 20 (74%) | 0.7940 |
| Clinical signs/symptoms | |||
| Torsade de pointes | 18 (39%) | 0 | N/A |
| Cardiac reanimation | 15 (32.6%) | 0 | N/A |
| Syncope | 15 (32.6%) | 0 | N/A |
| Severe dizziness | 6 (13%) | 0 | N/A |
| Near-fainting | 10 (21.7%) | 0 | N/A |
| Co-morbidities and risk factors | |||
| Chronic kidney disease | 5 (10.8% ) | 3 (11% ) | 0.7217 |
| Chronic heart failure | 9 (19.5%) | 6 (22.2%) | 0.9770 |
| Hypokalemia | 19 (41.3%) | 13 (48%) | 0.7455 |
| Bradycardia | 9 (19.5%) | 5 (18.5%) | 0.8429 |
| Alcoholism | 9 (19.5%) | 6 (22.2%) | 0.9770 |
| QT prolongation | Symptoms | No symptoms |
|---|---|---|
| All drugs involved in any iatrogenic cases of prolongation of QTc interval, that had come to the observation of the two clinical institutes engaged in the retrospective study in the decade 2007 - 2017 are represented. Information is provided about the existence of a possible associated symptomatology. Of note, the involved 35 drugs were all marked by a “certain” or “probable” drug causality assessment, according to the respective definitions given in the text of Methods. Among the 73 validated adverse drug reactions consisting of drug-induced QTc lengthening, in 23 cases our retrospective investigation led to finding of two candidate drugs, as being equally involved as causative factors in the QTc prolongation. | ||
| Metoclopramide | 3 | 0 |
| Amiodarone | 3 | 7 |
| Dronedarone | 0 | 2 |
| Flecainide | 1 | 1 |
| Fluvastatin | 1 | 1 |
| Fosinopril + hydrochlorothiazide | 1 | 1 |
| Hydrochlorothiazide | 1 | 2 |
| Sotalol | 0 | 2 |
| Terlipressin | 4 | 0 |
| Aciclovir | 2 | 1 |
| Ceftriaxon | 1 | 2 |
| Ciprofloxacin | 4 | 4 |
| Clarithromycin | 1 | 1 |
| Cotrimoxazol | 0 | 1 |
| Ganciclovir | 1 | 0 |
| Levofloxacin | 1 | 4 |
| 5-Fluorouracil | 1 | 0 |
| Oxaliplatin | 1 | 0 |
| Trastuzumab | 0 | 1 |
| Tacrolimus | 2 | 0 |
| Fentanyl | 1 | 0 |
| Tramadol | 0 | 1 |
| Carbamazepin | 1 | 0 |
| Valproic acid | 1 | 0 |
| Citalopram | 6 | 0 |
| Escitalopram | 4 | 0 |
| Fluoxetine | 2 | 0 |
| Haloperidol | 6 | 0 |
| Imipramine | 1 | 0 |
| Mirtazapine | 2 | 0 |
| Olanzapine | 4 | 0 |
| Promethazin | 1 | 0 |
| Quetiapin | 2 | 1 |
| Thioridazine | 1 | 0 |
| Methadone | 2 | 0 |
| 62 | 34 | |
| Dependent (Y) variable: event or symptom ( composite end-point) | |||||
|---|---|---|---|---|---|
| Exposure variables | Coefficient | Std. error | Odds ratio | 95% CI | P |
| In this series of patients with iatrogenic diLQT retrospectively collected and included in bivariate analysis, using as exposure variables both the duration of the QTc (continuous variable) and the duration of the QTc > 500 ms (categorical variable), the above-mentioned electrocardiographic abnormality is not independently associated with increased risk of events (cardiac arrest from TdP or ventricular fibrillation) or symptoms (dizziness, near-syncope, syncope). Please see also the Discussion for possible reasons underlying this finding. QTc: QT corrected for heart rate; Std. error: standard error; CI: confidence interval; diLQTc: drug-induced long QTc. | |||||
| QTc interval duration on surface ECG | -0.00109 | 0.0074 | 0.998 | 0.984 to 1.013 | 0.8821 |
| Presence of QTc > 500 ms on surface ECG | -0.62763 | 0.6063 | 0.533 | 0.162 to 1.752 | 0.3006 |