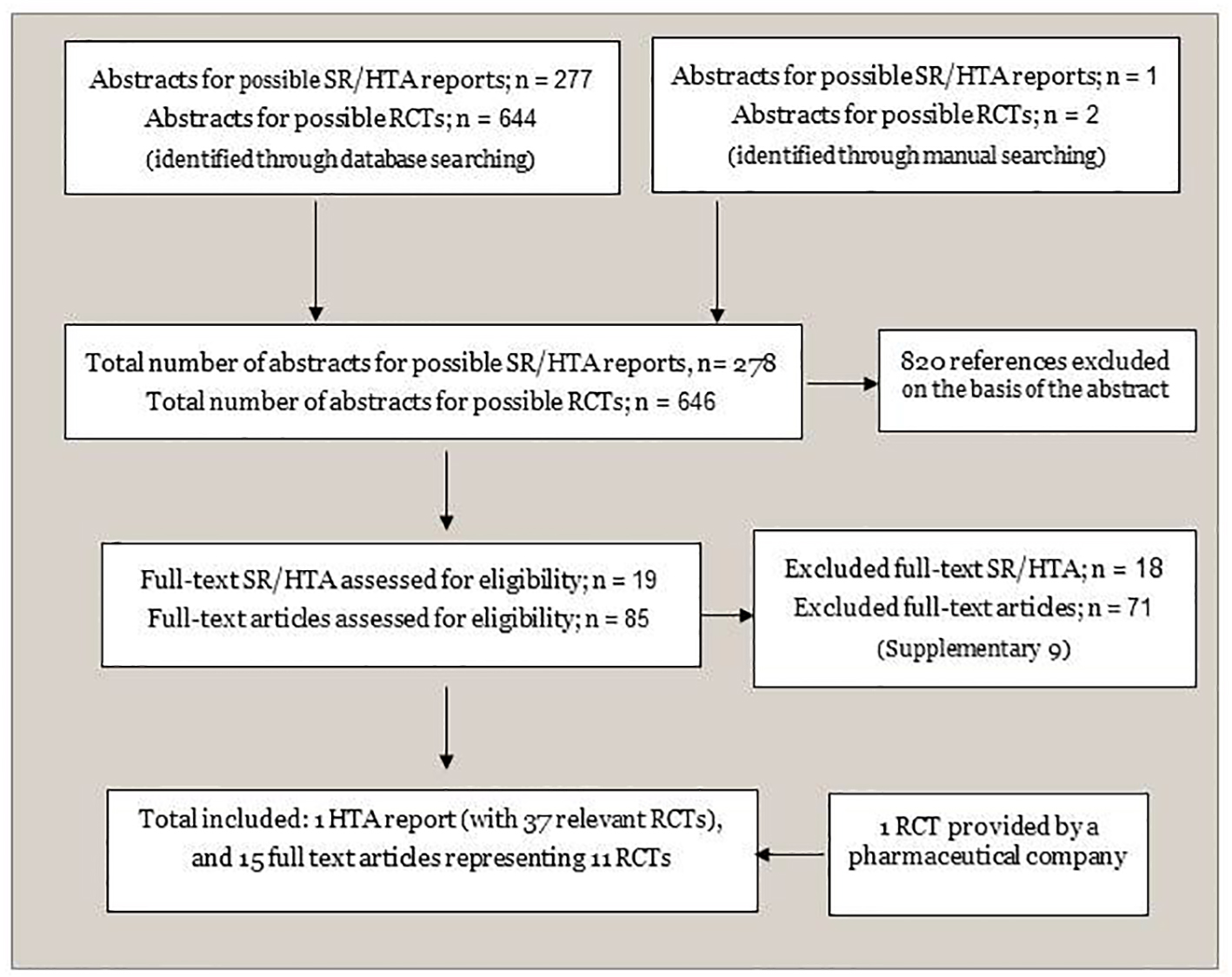
Figure 1. Flowchart of identification and selection of documentation. SR: systematic review; HTA: health technology assessment; RCT: randomized controlled trial.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 2, February 2018, pages 88-105
A Multiple Treatment Comparison of Eleven Disease-Modifying Drugs Used for Multiple Sclerosis
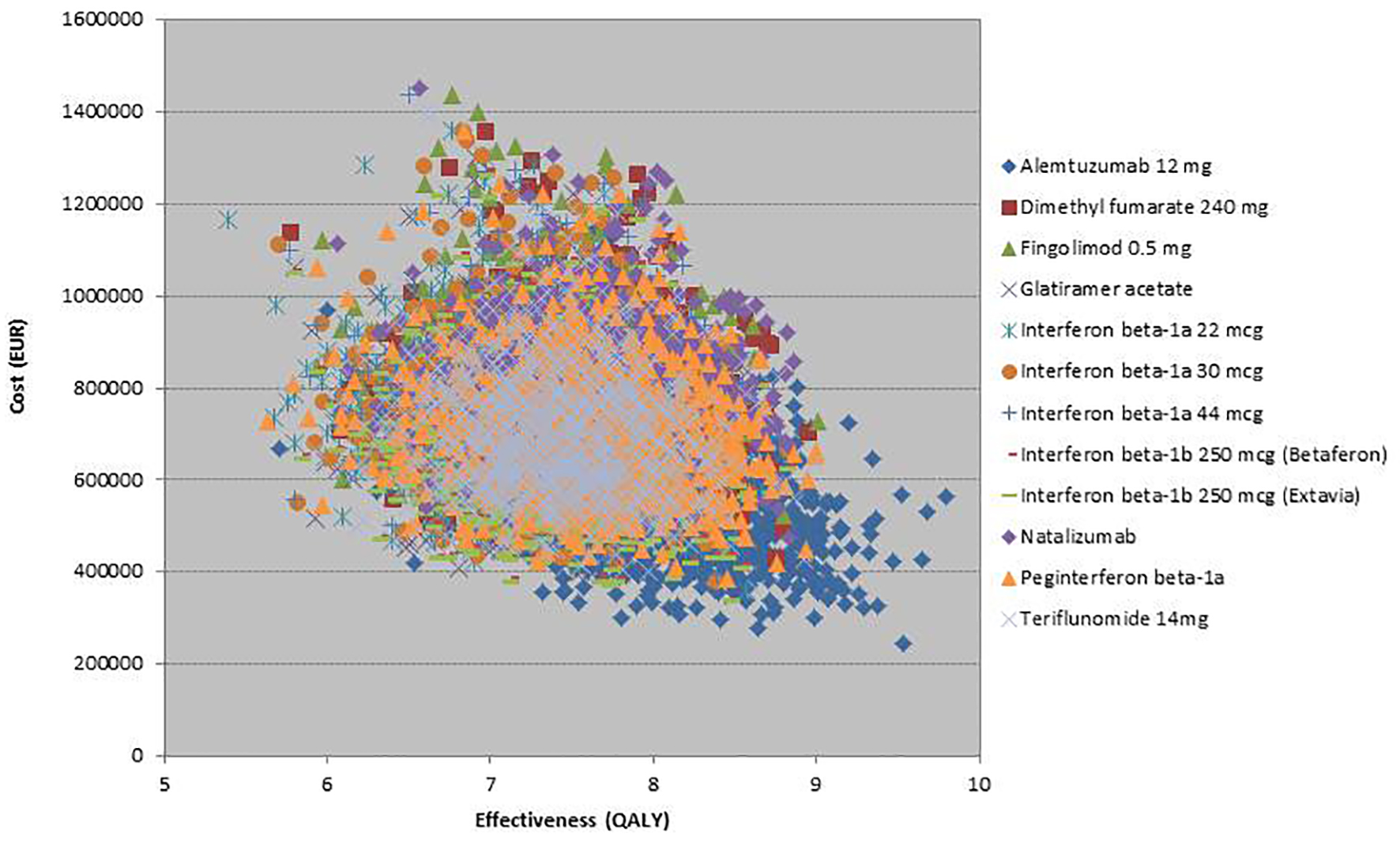
Figures

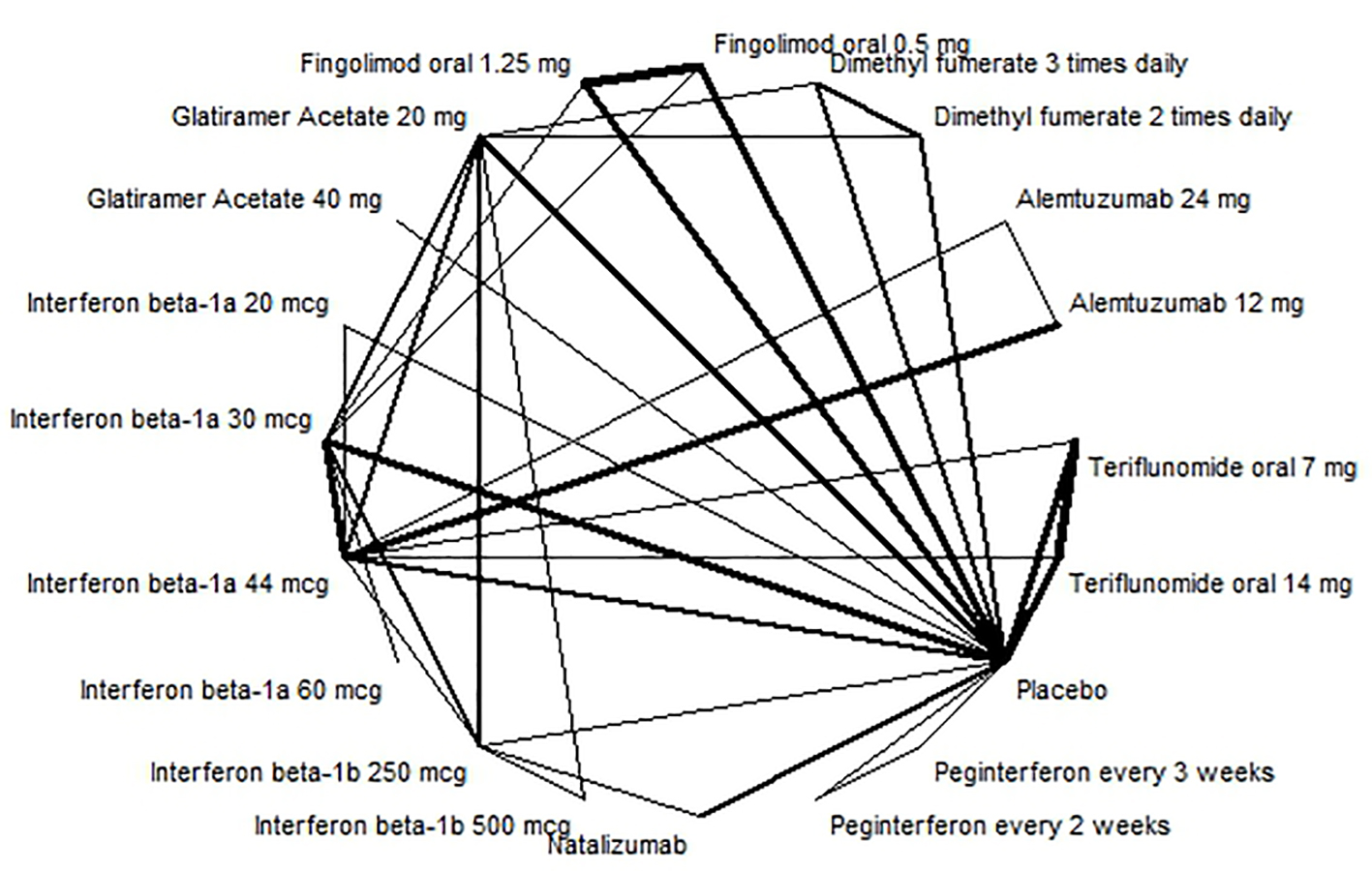
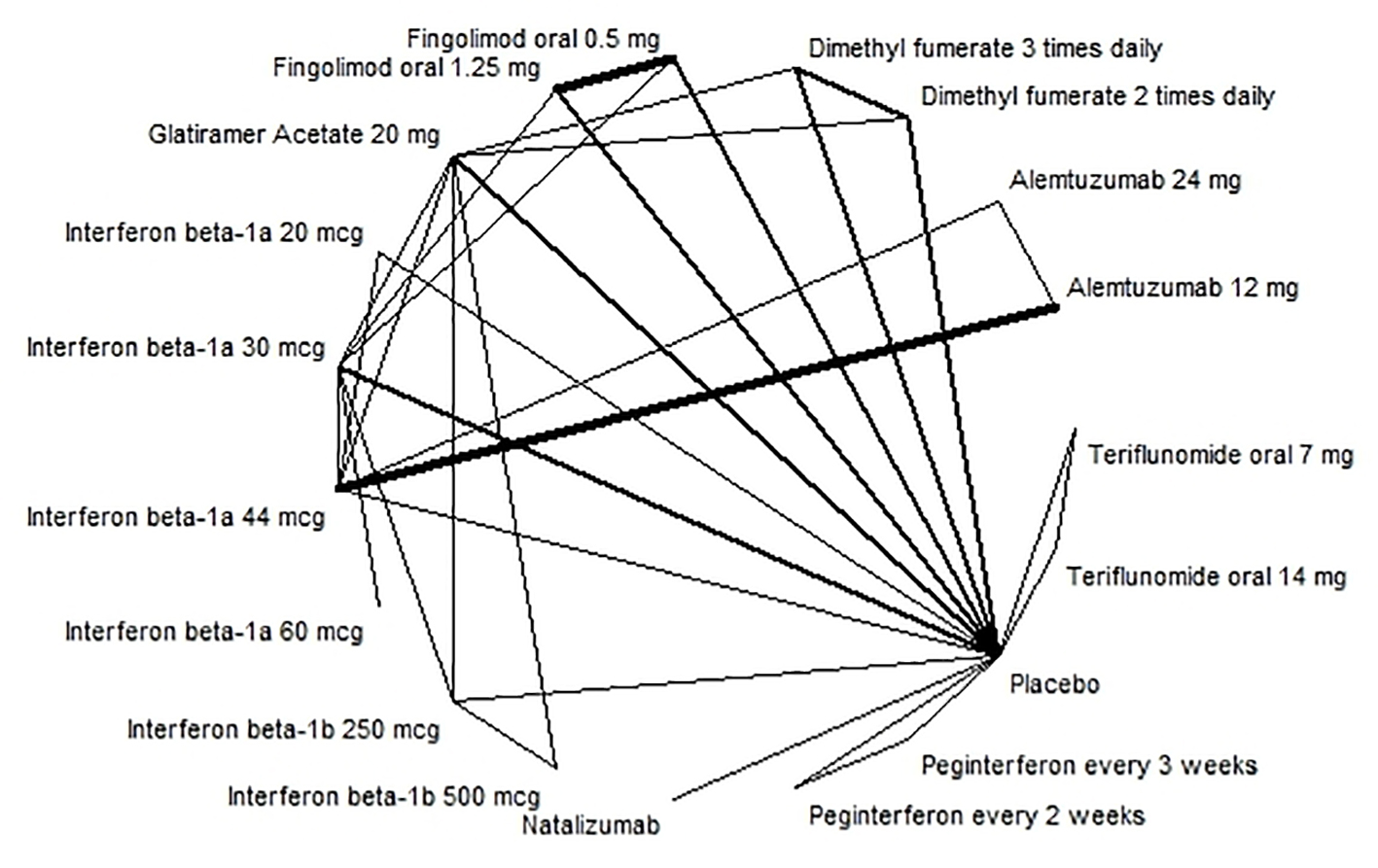
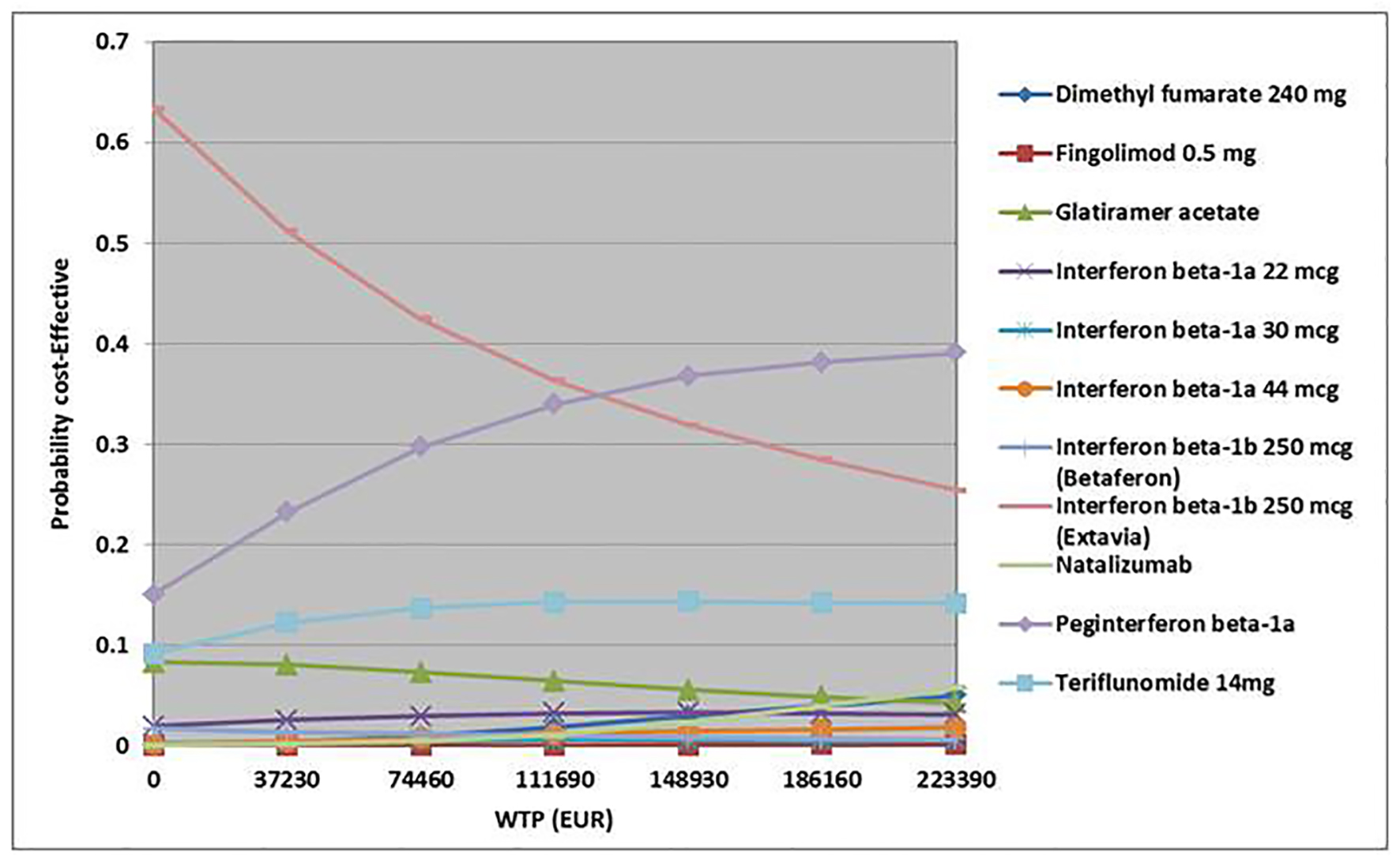
Tables
| Interventions | Administration form and recommended dose |
|---|---|
| mg: milligram; IV: intravenous; h: hour; mL: millilitre; μg: microgram; IU: International Units; IM: intra muscular. | |
| Alemtuzumab (Lemtrada) | 12 mg concentrate for solution for infusion 12 mg/day for 5 consecutive days, then after 12 months: 12 mg/day for 3 consecutive days. Diluted and IV over approximately 4 h |
| Dimethyl fumarate (Tecfidera) | 120 or 240 mg gastro-resistant hard capsules 240 mg twice daily |
| Fingolimod (Gilenya) | 0.5 mg hard capsules 0.5 mg once daily |
| Glatiramer acetat (Copaxone) | 20 mg/mL solution for injection, pre-filled syringe 20 mg of glatiramer acetate (one pre-filled syringe), administered as a subcutaneous injection once daily 40 mg of glatiramer acetate administered three times weekly |
| Interferon beta-1a (Avonex) | 30 µg (6 million IU) powder and solvent for solution for injection 30 µg (1 mL solution), by intramuscular (IM) injection once a week |
| Interferon beta-1a (Rebif) | 22 µg (6 million IU) solution for injection in pre-filled syringe 44 µg given three times per week by subcutaneous injection |
| Peg-interferon beta-1a (Plegridy) | 125 µg injected subcutaneously every 2 weeks |
| Interferon beta-1b (Betaferon) | 250 µg (8.0 million IU)/mL, powder and solvent for solution for injection (300 µg (9.6 million IU) per vial) 250 µg (8.0 million IU), contained in 1 mL of the reconstituted solution, to be injected subcutaneously every other day |
| Interferon beta-1b (Extavia) | See: interferon beta-1b (Betaferon) above |
| Natalizumab (Tysabri) | 300 mg concentrate for solution for infusion 300 mg by IV over approximately 1 h, once every 4 weeks |
| Teriflunomide (Aubagio) | 14 mg film-coated tablets 14 mg once daily, swallowed whole with some water |
| Definition | Value | Source/note | |||
|---|---|---|---|---|---|
| EDSS: expanded disability status scale; SPMS: secondary-progressive multiple sclerosis; RR: relative risk; CI: confidence interval; SE: standard error; PML: progressive multifocal leukoencephalopathy. aFor the other probabilities used in the model, see Supplementary materials 4 and 5 (www.jocmr.org). bPer person-year. cGlatiramer acetate 40 mg RR: 0.66 (0.52 to 0.82). dWe did not find any documentation for glatiramer acetate 40 mg. eThe majority of patients receiving alemtuzumab would not need new treatment after 5 years treatment. It was assumed that 20% of patients need extra treatment (12 mg/day for 3 days) [29]. fGlatiramer acetate 40 mg/mL three times per week: Annual drug cost was estimated to be EUR 9,732. gWe used the price for the products (pre-filled syringe and auto-injector pen) that had 85% of Rebif 44- market in the recent years in Norway (2013 - 2015). hIt was assumed that the average length of mild or moderate relapse and severe relapse would be 45 and 90 days, respectively [24, 25]. iAll costs were updated to 2015 costs. jAssumed fixed utility decrement over the corresponding RRMS EDSS state utility values. kIt was estimated based on the data reported by Orme et al [27] and Prosser et al [25]. | |||||
| Transition probabilitiesa | EDSS score | Progression rates within RRMS health statesb (variance) | Progression rates from RRMS to SPMSb (variance) | Progression rates within SPMS health statesb (variance) | [23, 24] |
| 0 | 0.144 (0.00007) | 0.004 (0.000002) | |||
| 1 | 0.075 (0.00003) | 0.002 (0.000001) | |||
| 2 | 0.152 (0.00006) | 0.029 (0.000012) | 0.370 (0.00370) | ||
| 3 | 0.272 (0.00025) | 0.102 (0.000094) | 0.385 (0.00129) | ||
| 4 | 0.450 (0.00166) | 0.199 (0.000735) | 0.594 (0.00280) | ||
| 5 | 0.485 (0.00213) | 0.256 (0.001126) | 0.349 (0.00088) | ||
| 6 | 0.283 (0.00104) | 0.184 (0.000676) | 0.241 (0.00029) | ||
| 7 | 0.342 (0.00450) | 0.237 (0.000312) | 0.186 (0.00024) | ||
| 8 | 0.105 (0.00139) | 0.066 (0.000866) | 0.107 (0.00015) | ||
| 9 | 0.167 (0.02778) | 0.167 (0.027778) | 0.093 (0.00038) | ||
| Efficacy estimate (Mot placebo) | Interventions | RR of annual relapse rate (95% CI) | RR of disability progression (95% CI) | Based on the results of our systematic review (Table 3) | |
| Alemtuzumab 12 mg (Lemtrada) | 0.29 (0.23 - 0.35) | 0.36 (0.16 - 0.74) | |||
| Dimethyl fumarate 240 mg (Tecifidera) | 0.50 (0.42 - 0.60) | 0.65 (0.49 - 0.85) | |||
| Fingolimod 0.5 mg (Gilenya) | 0.46 (0.39 - 0.54) | 0.71 (0.55 - 0.90) | |||
| Glatiramer acetate 20 mg (Copaxone) | 0.65 (0.59 - 0.73)c | 0.78 (0.63 - 0.96)d | |||
| Interferon beta-1a 30 µg (Avonex) | 0.82 (0.73 - 0.91) | 0.80 (0.65 - 0.99) | |||
| Interferon beta-1a 22 µg (Rebif) | 0.69 (0.57 - 0.83) | 0.84 (0.61 - 1.19) | |||
| Interferon beta-1a 44 µg (Rebif) | 0.64 (0.56 - 0.72) | 0.77 (0.60 - 1.01) | |||
| Interferon beta-1b 250 µg (Betaferon) | 0.66 (0.57 - 0.76) | 0.72 (0.54 - 0.92) | |||
| Interferon beta-1b 250 µg (Extavia) | 0.66 (0.57 - 0.76) | 0.72 (0.54 - 0.92) | |||
| Natalizumab 300 mg (Tysabri) | 0.30 (0.24 - 0.36) | 0.59 (0.42 - 0.84) | |||
| Peg-interferon beta-1a 125 µg (Plegridy) | 0.65 (0.49 - 0.85) | 0.61 (0.36 - 0.98) | |||
| Teriflunomide 14 mg (Aubagio) | 0.67 (0.58 - 0.77) | 0.73 (0.51 - 1.05) | |||
| Costs per patients per year in EURi (EUR 1.00 ≈ NOK 8.9530) | Interventions | Annual drug costs | Based on drug procurement cooperation price (LIS) | ||
| Alemtuzumab 12 mg (Lemtrada)e | 35,607 (5 days first year); 21,364 (3 days second year) | ||||
| Dimethyl fumarate 240 mg (Tecifidera) | 18,839 | ||||
| Fingolimod 0.5 mg (Gilenya) | 22,023 | ||||
| Glatiramer acetate 20 mg (Copaxone)f | 9,759 | ||||
| Interferon beta-1a 30 µg (Avonex) | 11,648 | ||||
| Interferon beta-1a 22 µg (Rebif) | 10,204 | ||||
| Interferon beta-1a 44 µg (Rebif)g | 12,929 | ||||
| Interferon beta-1b 250 µg (Betaferon) | 7,407 | ||||
| Interferon beta-1b 250 µg (Extavia) | 6,709 | ||||
| Natalizumab 300 mg (Tysabri) | 21,428 | ||||
| Peg-interferon beta-1a 125 µg (Plegridy) | 12,808 | ||||
| Teriflunomide 14 mg (Aubagio) | 11,769 | ||||
| Interventions | Monitoring costs | Based on drug procurement cooperation (LIS) and clinical expert opinion | |||
| 1 year | 2 years | Beyond 2 years | |||
| Alemtuzumabe (Lemtrada) | 2,539 | 1,628 | 928 (3 - 5 years) 790 (+5 years) | ||
| Dimethyl fumarate (Tecifidera) | 1,290 | 790 | 790 | ||
| Fingolimod (Gilenya) | 2,001 | 790 | 790 | ||
| Glatiramer acetate (Copaxone) | 1,290 | 790 | 790 | ||
| Interferon beta-1a 30 µg (Avonex) | 2,152 | 1,652 | 790 | ||
| Interferon beta-1a 22 µg (Rebif) | 2,152 | 1,652 | 790 | ||
| Interferon beta-1a 44 µg (Rebif) | 2,152 | 1,652 | 790 | ||
| Interferon beta-1b (Betaferon) | 2,152 | 1,652 | 790 | ||
| Interferon beta-1b (Extavia) | 2,152 | 1,652 | 790 | ||
| Natalizumab (Tysabri) | 3,713 | 3,097 | 3,097 | ||
| Peg-interferon beta-1a (Plegridy) | 2,152 | 1,652 | 790 | ||
| Teriflunomide (Aubagio) | 1,440 | 840 | 840 | ||
| EDSS score | Costs associated to different EDSS states | [26] | |||
| 0 | 2,016 | ||||
| 1 | 4,122 | ||||
| 2 | 5,730 | ||||
| 3 | 14,090 | ||||
| 4 | 16,481 | ||||
| 5 | 36,830 | ||||
| 6 | 63,099 | ||||
| 7 | 76,982 | ||||
| 8 | 154,171 | ||||
| 9 | 155,661 | ||||
| Costs per relapseh | |||||
| Mild/moderate | 2,447 | ||||
| Severe | 4,894 | ||||
| Utility weight | Health state/events | Utility weight (95% CI) | [27] Disability associated with PML: [28] | ||
| EDSS 0 | 0.870 (0.782 to 0.958) | ||||
| EDSS 1 | 0.799 (0.617 to 0.981) | ||||
| EDSS 2 | 0.705 (0.523 to 0.886) | ||||
| EDSS 3 | 0.574 (0.384 to 0.763) | ||||
| EDSS 4 | 0.610 (0.428 to 0.791) | ||||
| EDSS 5 | 0.518 (0.338 to 0.698) | ||||
| EDSS 6 | 0.460 (0.0277 to 0.641) | ||||
| EDSS 7 | 0.297 (0.112 to 0.481) | ||||
| EDSS 8 | -0.049 (-0.235 to 0.138) | ||||
| EDSS 9 | -0.195 (-0.428 to 0.039) | ||||
| SPMSj | -0.045 (-0.079 to -0.014) | ||||
| Disutility associated with mild or moderate relapse | -0.071 (-0.096 to -0.046) | ||||
| Disutility associated with severe relapsek | -0.236 (-0.295 to -0.174) | ||||
| Disability associated with PML | -0.40 (-0.30 to -0.50) | ||||
| Interventions | Annual relapse | Disability progression | ||
|---|---|---|---|---|
| RR (95% CI) | GRADE | RR (95% CI) | GRADE | |
| RR: relative ratio; CI: confidence interval; SC: subcutaneous; IM: intra muscular; q.d.: once daily, q.w.: once weekly; t.i.w.: three times weekly; 2.i.d: two times daily; t.i.d: three times daily; 1/2 w: once every 2 weeks; 1/4 w: once every 4 weeks; NA: not available. | ||||
| Alemtuzumab 24 mg IV q.d. | 0.16 (0.1 to 0.25) | Low | 0.40 (0.27 to 0.60) | Low |
| Alemtuzumab 12 mg IV q.d. | 0.29 (0.23 to 0.35) | High | 0.36 (0.16 to 0.74) | Very low |
| Natalizumab | 0.3 (0.24 to 0.36) | Moderate | 0.59 (0.42 to 0.84) | Moderate |
| Fingolimod oral 1.25 mg | 0.45 (0.39 to 0.53) | High | 0.71(0.56 to 0.90) | High |
| Fingolimod oral 0.5 mg | 0.46 (0.39 to 0.54) | High | 0.71 (0.55 to 0.90 | High |
| Dimethyl fumarate 240 mg 2.i.d. | 0.5 (0.42 to 0.6) | High | 0.65 (0.49 to 0.85) | High |
| Dimethyl fumarate 240 mg t.i.d. | 0.5 (0.42 to 0.6) | High | 0.68 (0.52 to 0.89) | High |
| Interferon beta-1b 500 µg SC 1/2 d | 0.62 (0.51 to 0.74) | Moderate | 0.79 (0.56 to 1.10) | Low |
| Interferon beta-1a 44 µg | 0.64 (0.56 to 0.72) | High | 0.77 (0.60 to 1.01) | Low |
| Peg-interferon beta-1a 125 µg 1/2 w | 0.65 (0.49 to 0.85) | High | 0.61 (0.36 to 0.98) | Low |
| Glatiramer acetate 20 mg | 0.65 (0.59 to 0.73) | High | 0.78 (0.63 to 0.96) | Low |
| Glatiramer acetate 40 mg | 0.66 (0.52 to 0.82) | High | NA | NA |
| Interferon beta-1b 250 µg | 0.66 (0.57 to 0.76) | Moderate | 0.2 (0.54 to 0.92) | Low |
| Teriflunomide oral 14 mg | 0.67 (0.58 to 0.77) | High | 0.73 (0.51 to 1.05) | Low |
| Interferon beta-1a 22 µg | 0.69 (0.57 to 0.83) | Moderate | 0.84 (0.61 to 1.19 | Low |
| Peg-interferon beta-1a 125 µg 1/4 w | 0.73 (0.56 to 0.95) | High | 0.62 (0.38 to 1.01) | Low |
| Teriflunomide oral 7 mg | 0.77 (0.68 to 0.9) | High | 0.80 (0.55 to 1.13) | Low |
| Interferon beta-1a 30 µg | 0.82 (0.73 to 0.91) | High | 0.80 0.65 to 0.99) | Moderate |
| Interferon beta-1a 60 µg IM q.w. | 0.86 (0.7 to 1.06) | High | NA | NA |
| Drugs | Total costs (EUR) | Effects (QALYs) | Incremental cost (EUR) | Incremental effect (QALYs) | ICER (EUR/QALY) |
|---|---|---|---|---|---|
| QALY: quality-adjusted life year; ICER: incremental cost-effectiveness ratio. aThe results are rounded in accordance to the calculations in the probabilistic model, which operates with several decimals. bBased on the effect estimates and the annual drug costs, it is highly probable that glatiramer acetate 40 mg three times/week will be as cost-effective as glatiramer acetate 20 mg /day (given all the other parameters are the same). | |||||
| Alemtuzumab (Lemtrada) | 547,068 | 8.05 | Dominant | ||
| Interferon beta-1b (Extavia) | 673,690 | 7.40 | 126,622 | -0.64 | Dominated by alemtuzumab |
| Interferon beta-1b (Betaferon) | 680,013 | 7.40 | 132,945 | -0.64 | Dominated by alemtuzumab |
| Glatiramer acetate 20 mg (Copaxone)b | 698,506 | 7.31 | 151,438 | -0.73 | Dominated by alemtuzumab |
| Peg-interferon beta-1a (Plegridy) | 704,857 | 7.56 | 157,789 | -0.48 | Dominated by alemtuzumab |
| Teriflunomide (Aubagio) | 707,862 | 7.38 | 160,794 | -0.67 | Dominated by alemtuzumab |
| Interferon beta-1a 22 µg (Rebif) | 725,854 | 7.21 | 178,786 | -0.84 | Dominated by alemtuzumab |
| Interferon beta-1a 30 µg (Avonex) | 729,802 | 7.27 | 182,734 | -0.77 | Dominated by alemtuzumab |
| Interferon beta-1a 44 µg (Rebif) | 734,347 | 7.32 | 187,279 | -0.72 | Dominated by alemtuzumab |
| Dimethyl fumarate (Tecifidera) | 749,222 | 7.52 | 202,154 | -0.52 | Dominated by alemtuzumab |
| Natalizumab (Tysabri) | 779,977 | 7.63 | 232,909 | -0.41 | Dominated by alemtuzumab |
| Fingolimod (Gilenya) | 786,464 | 7.43 | 239,396 | -0.62 | Dominated by alemtuzumab |