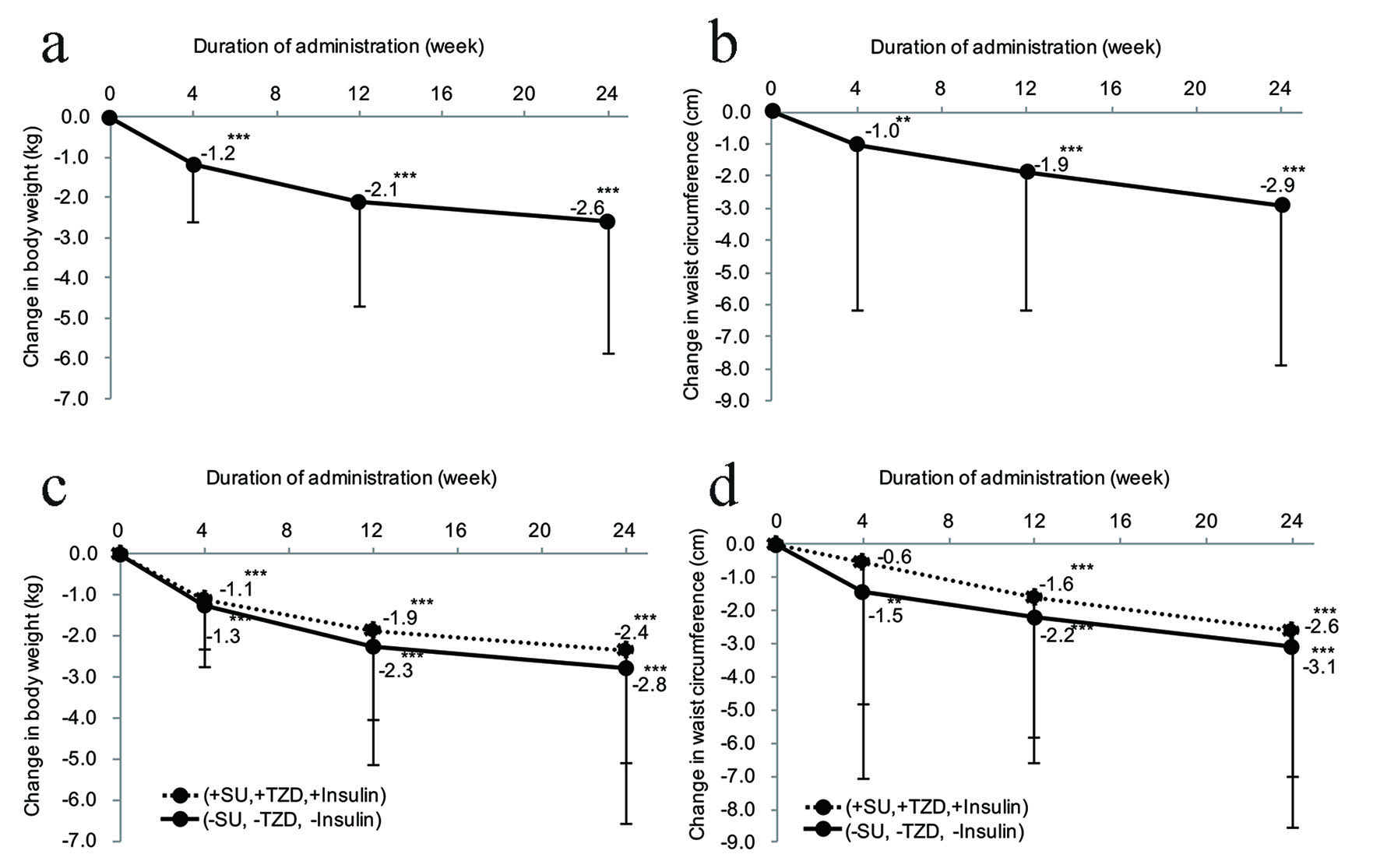
Figure 1. Effect of ipragliflozin on body weight and waist circumference. (a) Change in body weight after the start of ipragliflozin therapy (n = 295). (b) Change in waist circumference after the start of ipragliflozin therapy (n = 334). (c) Stratified analysis of the body weight changes in patients receiving ipragliflozin concomitantly with an SU, insulin, or TZD (n = 152) and patients receiving ipragliflozin without concomitant use of these drugs (n = 143). (d) Stratified analysis of the waist circumference changes in patients receiving ipragliflozin concomitantly with an SU, insulin, or TZD (n = 171) and patients receiving ipragliflozin without concomitant use of these drugs (n = 163). (e) Changes in body fat mass and fat-free body mass measured by a body composition analyzer from the start of ipragliflozin administration (body fat mass, n = 295; fat-free body mass, n = 295). (f) Changes in body composition measured by a body composition analyzer from the initiation of ipragliflozin treatment. Significant differences in week 4, week 12, and week 24 relative to the start of administration: ***P < 0.001; **P < 0.01; *P < 0.05, respectively. SU: sulfonylurea; TZD: thiazolidinedione.