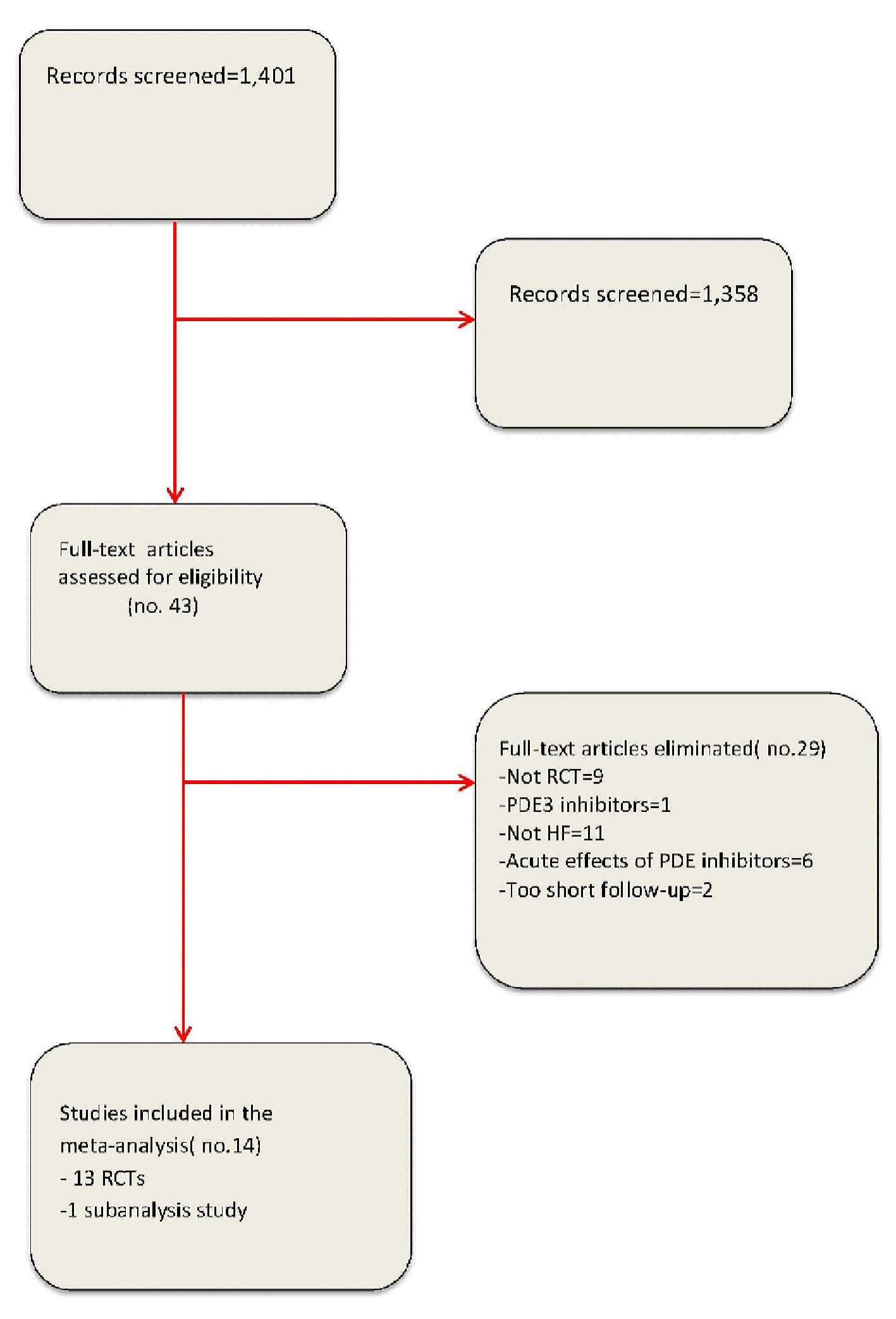
Figure 1. Flow diagram for meta-analysis according to PRISMA statement.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 6, June 2017, pages 488-498
Phosphodiesterase-5 Inhibitors Improve Clinical Outcomes, Exercise Capacity and Pulmonary Hemodynamics in Patients With Heart Failure With Reduced Left Ventricular Ejection Fraction: A Meta-Analysis
Figures

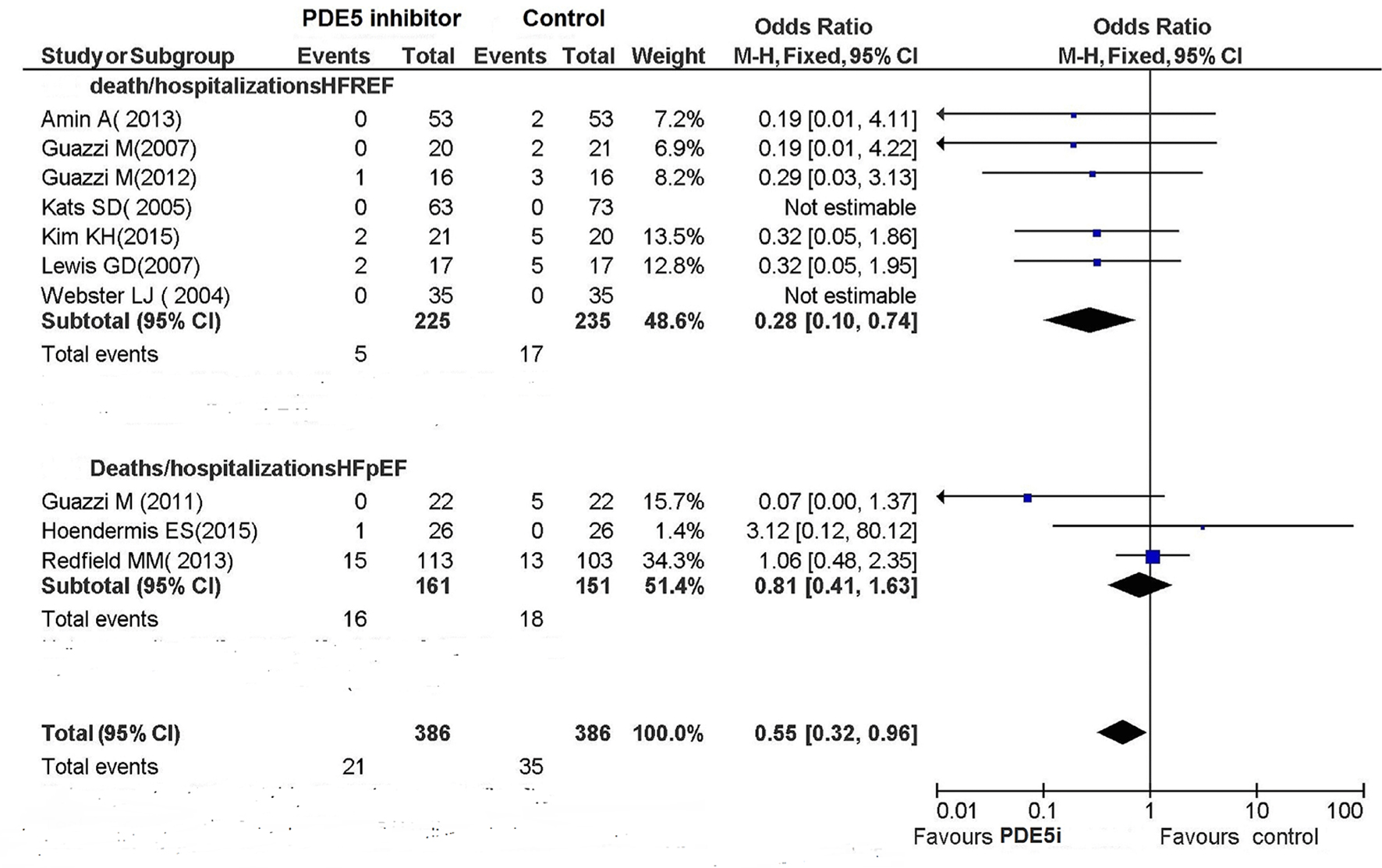
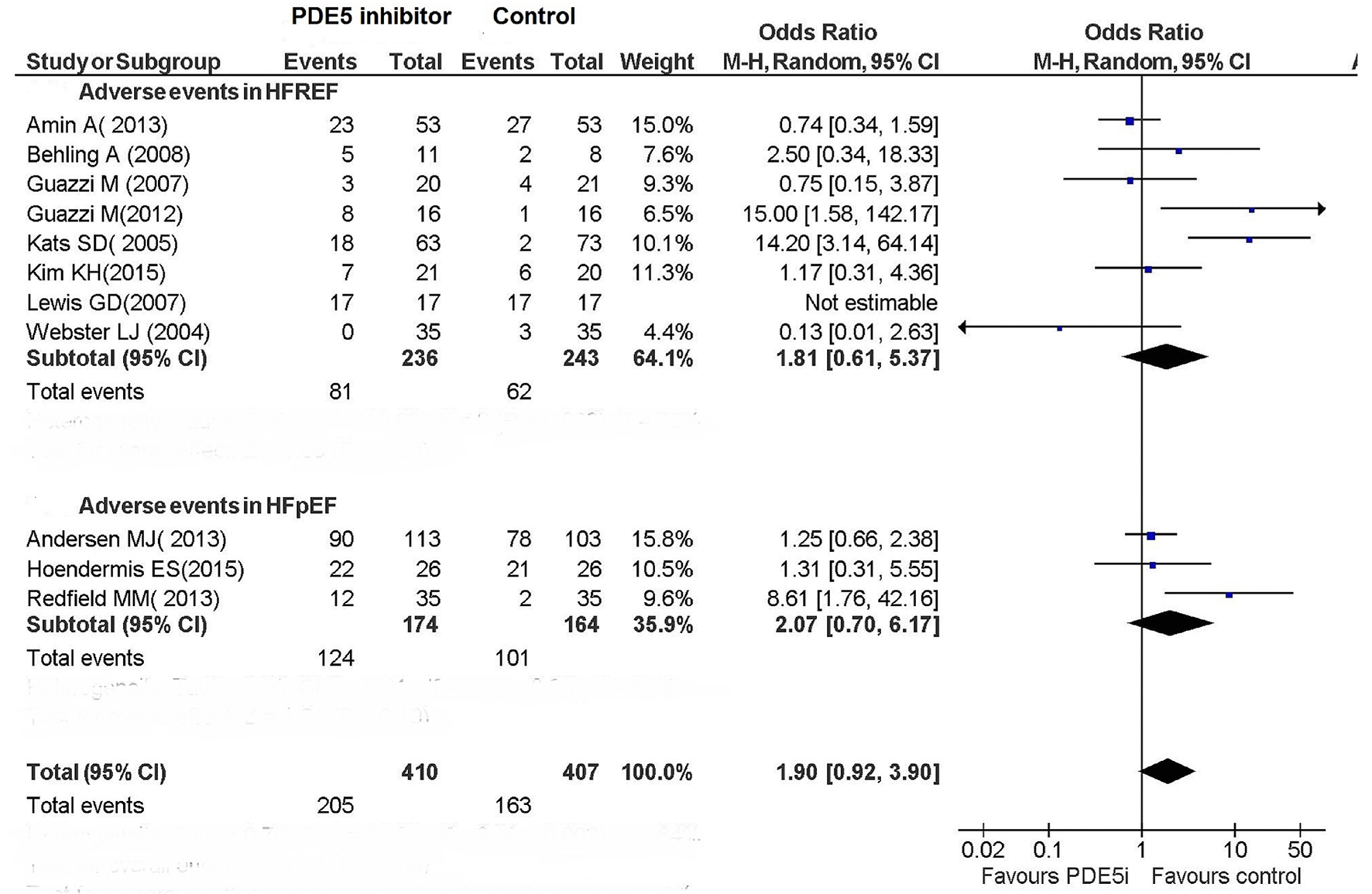
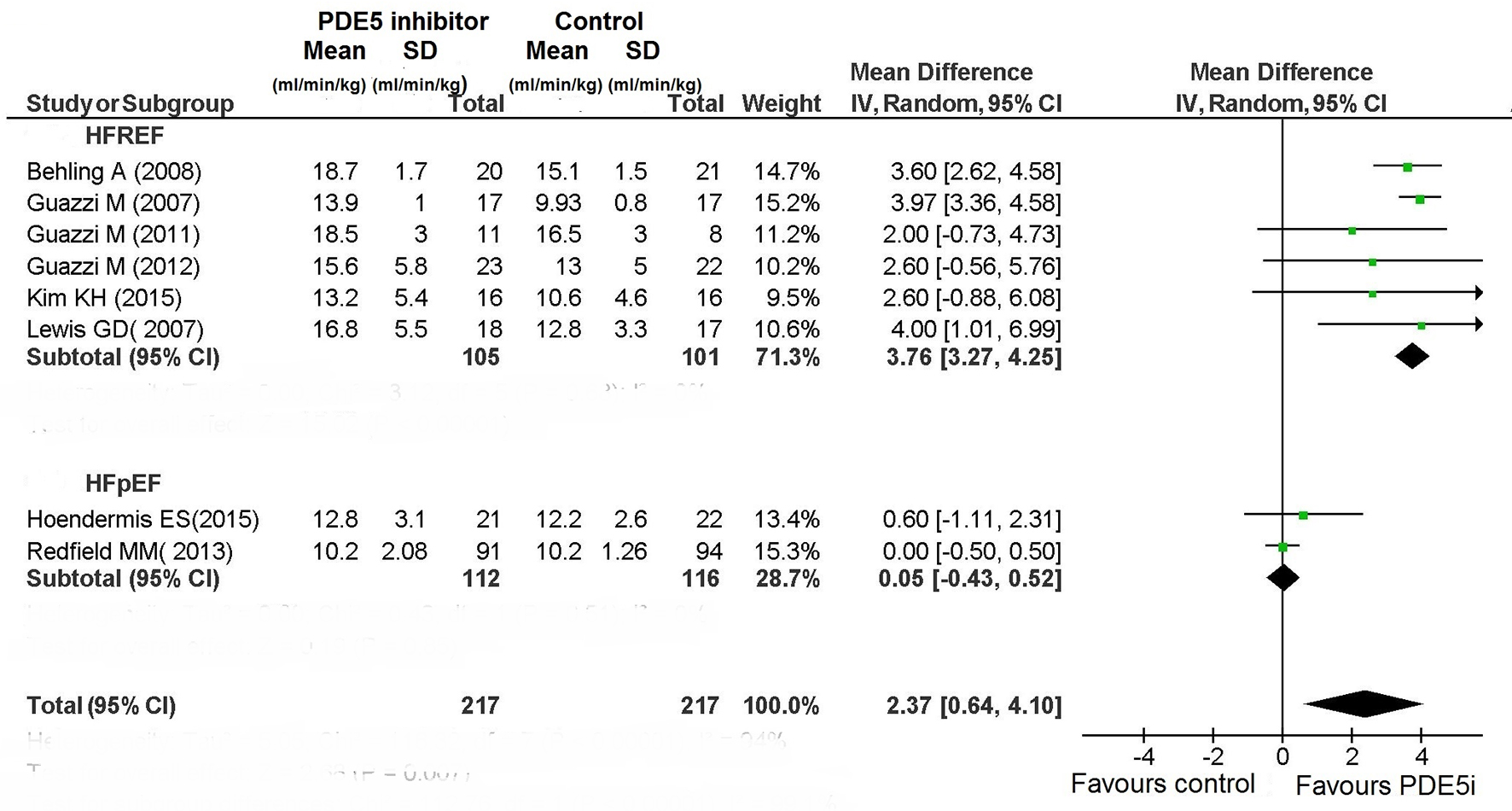
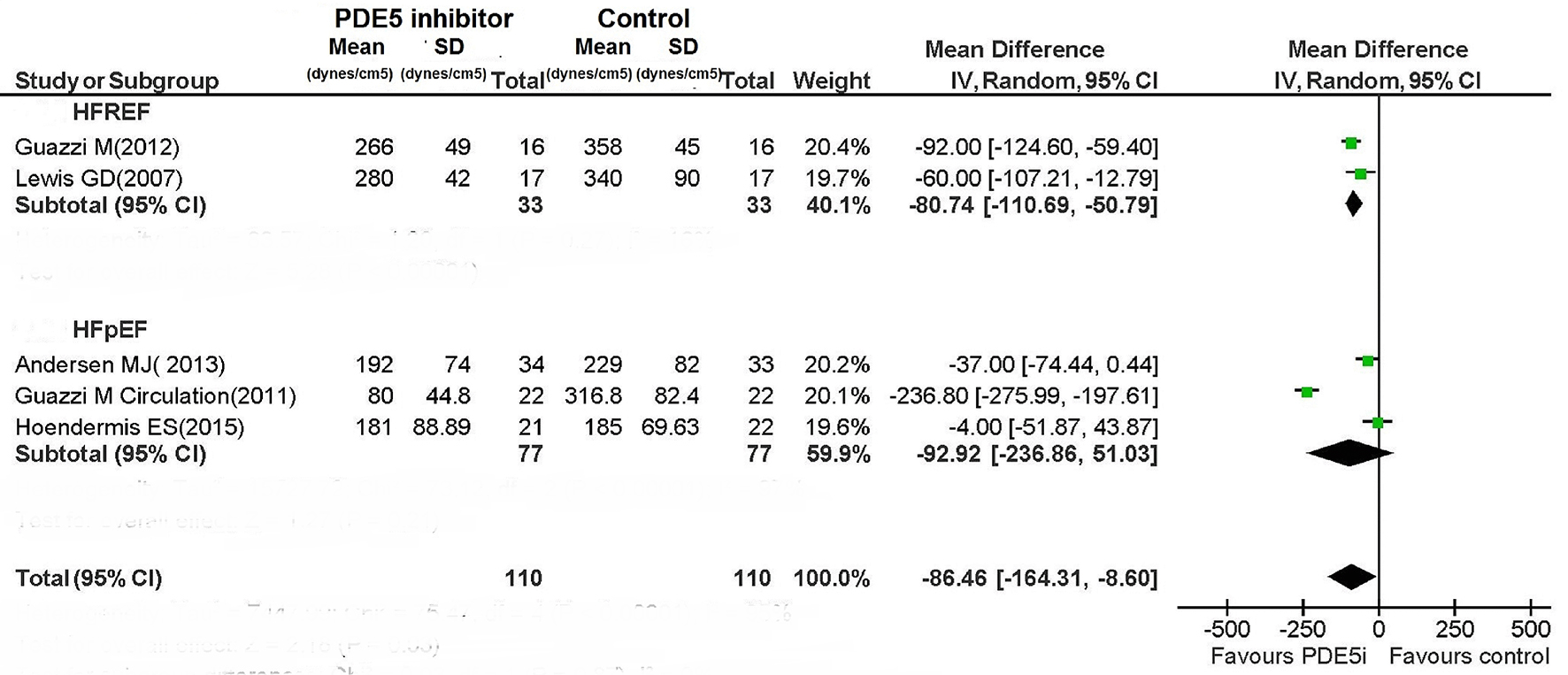
Tables
| Amin et al (2013) [7] | Andersen et al ( 2013) [8] | Behling et al (2008) [9] | Guazzi et al (Circ Heart Fail 2011) [10] | Guazzi et al (Circulation 2011) [11] | Guazzi et al (2012) [112] | Guazzi et al (2007) [13] | Hoendermis et al (2015) [14] | Katz et al (2005) [15] | Kim et al (2015) [16] | Lewis et al (2008) [17] | Lewis et al (2007) [3] | Redfield et al (2013) [18] | Webster et al (2004) [19] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| †Sub-analysis of Lewis et al. CHF: chronic heart failure; HFREF: heart failure with reduced left ventricular ejection fraction; HFPEF: heart failure with preserved left ventricular ejection fraction; PH: pulmonary hypertension; EOB: exercise oscillatory breathing; MI: myocardial infarction; NYHA: New York Heart Association; PDE5i: phosphodiesterase type 5 inhibitor; CPET: cardiopulmonary exercise test; echo-: echocardiography; FMD: flow-mediated dilatation; BNP: B-type natriuretic peptide; QoL: quality of life; BP: blood pressure; 6MWT: six-minute walking test; CMRI: cardiac magnetic resonance imaging. | ||||||||||||||
| Subjects randomized (n; PDE5i/placebo) | 53/53 | 35/35 | 11/8 | 23/22 | 22/22 | 16/16 | 23/23 | 21/22 | 60/72 | 21/20 | 15/15 | 17/17 | 113/103 | 35/35 |
| Drug name | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil | Udenafil | Sildenafil | Sildenafil | Sildenafil | Sildenafil |
| Drug dosage | 25 mg bid for first 2 weeks; 50 mg tid for next 10 weeks | 40 mg tid | 50 mg tid | 50 mg tid | 50 mg tid | 50 mg tid | 50 mg bid | 20 mg tid for first 2 weeks; 60 mg tid for next 10 weeks | 25/50/100 mg | 50 mg bid for first 4 weeks; 100 mg bid for next 8 weeks | 25 - 75 mg tid | 25 - 75 mg tid | 20 mg tid for first 12 weeks; 60 mg tid for next 12 weeks | 50 mg once daily |
| Inclusion criteria | HFREF | Diastolic dysfunction after MI | HFREF | HFREF | HFPEF with PH | HFREF with PH and EOB | HFREF | HFPEF with PH | CHF (HFREF) with ED | HFREF | HFREF with PH† | HFREF with PH | HFPEF | CHF (HFREF) |
| NYHA | II-III | - | I-III | II-III | II-IV | III-IV | II-III | II-III | I-III | II-IV | II-IV | II-IV | II-IV | II-III |
| LVEF | < 35% | ≥ 45% | ≤ 40% | < 40% | ≥ 50% | < 45% | ≤ 45% | ≥ 45% | ≤ 40% | ≤ 40% | < 40% | < 40% | ≥ 50% | - |
| Follow-up duration (months) | 3 | 2 | 1 | 12 | 12 | 12 | 6 | 3 | 3 | 3 | 3 | 3 | 6 | 1.5 |
| Outcome measures | BP, NYHA, 6MWT | Echo-, cardiac cath, CPET, 6MWT | Echo-, CPET, FMD | Echo-, CPET, BNP, QoL | Echo-, cardiac cath, QoL | CPET, cardiac cath | Echo-, CPET, FMD | Cardiac cath, CPET, Echo- | International index of erectile function | Echo-, CPET | Cardiac cath, CPET, ventriculo-graphy | CPET: peak VO2 | Echo-, CMRI, CPET, 6MWT | International index of erectile function |
| Amin et al (2013) [7] | Andersen et al (2013) [8] | Behling (2008) [9] | Guazzi et al (Circ Heart Fail 2011) [10] | Guazzi et al (Circulation 2011) [11] | Guazzi et al (2012) [12] | Guazzi et al (2007) [13] | Hoendermis et al (2015) [14] | Kim et al (2015) [16] | Lewis et al (2008) [17] | Lewis et al (2007) [3] | Redfield et al ( 2013) [18] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
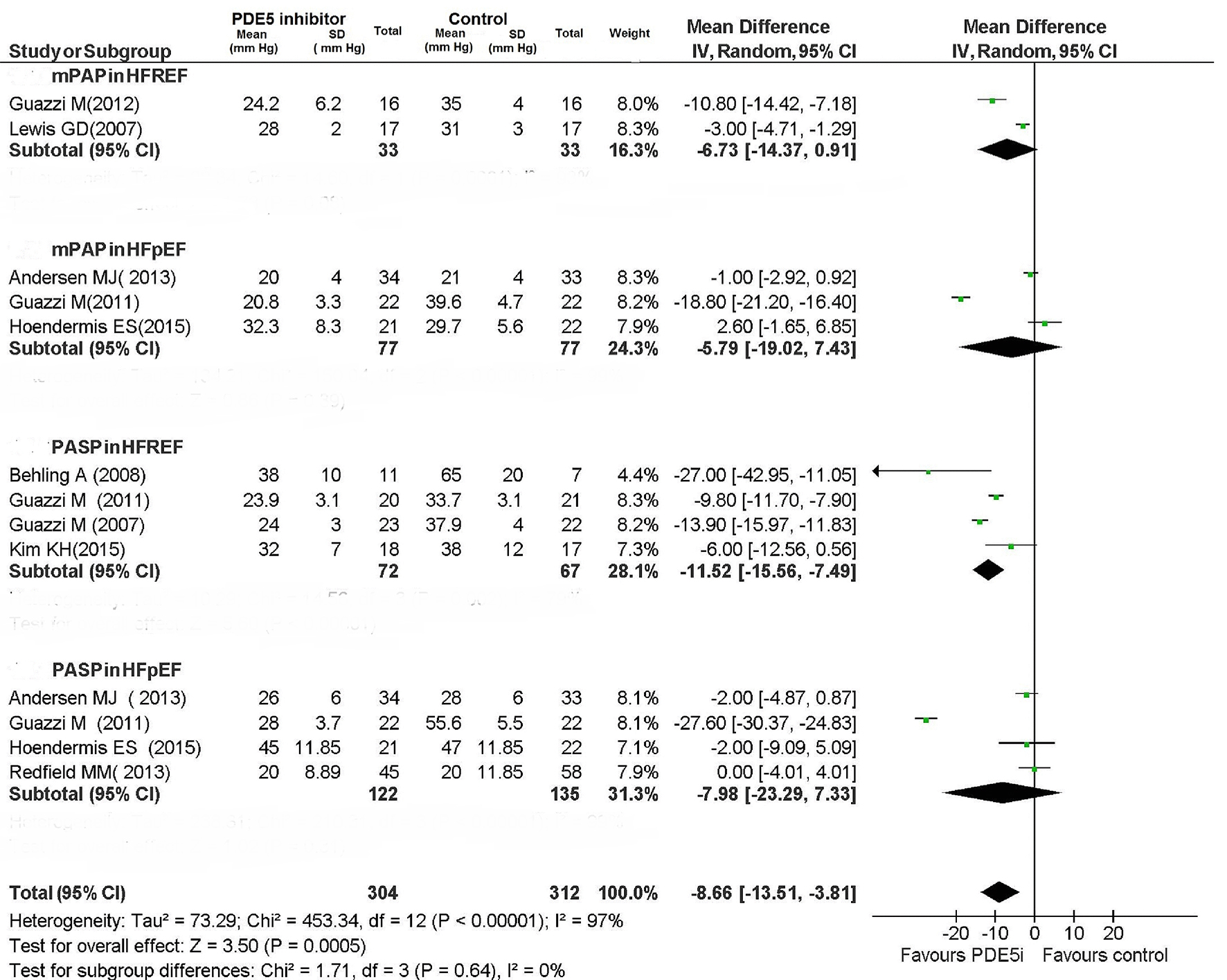
| Improvement in exercise capacity was evaluated based on the changes in peak VO2 and VE/VCO2 slope evidenced by cardiopulmonary exercise test, or based on 6MWT. Improvement in LV function was evaluated based on the changes in LVEF. Reduction in pulmonary pressures was evaluated based on the changes in mPAP, PCWP and PVR by means of cardiac cath, or using PASP derived from echocardiogram. *Converted from echocardiographic PASP by the following equation: mPAP (mm Hg) = (0.61 × PASP (mm Hg)) + 2 mm Hg [5]. †Sub-analysis of Lewis et al, 2007. HFREF: heart failure with reduced left ventricular ejection fraction; HFPEF: heart failure with preserved left ventricular ejection fraction; PH: pulmonary hypertension; EOB: exercise oscillatory breathing; MI: myocardial infarction; mPAP: mean pulmonary arterial pressure; dPAP: diastolic pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; TPG: transpulmonary gradient; DPG: diastolic pulmonary gradient; PVR: pulmonary vascular resistance; N/A: not applicable. | ||||||||||||
| Inclusion criteria | HFREF | Diastolic dysfunction after MI | HFREF | HFREF | HFPEF with PH | HFREF with PH and EOB | HFREF | HFPEF with PH | HFREF | HFREF with PH† | HFREF with PH | HFPEF |
| Pulmonary hemodynamic parameters | ||||||||||||
| mPAP (mm Hg; PDE5i/placebo) | - | 19/20 | 36.2/39.8* | 24.6/25.2* | 39/37 | 35/34 | 22.7/21.5* | 35/35 | 27/28.2* | 30/33 | 30/33 | 27/27* |
| dPAP (mm Hg; PDE5i/placebo) | - | 14/15 | - | - | 31.6/29.7 | - | - | 20/21 | - | - | - | - |
| PCWP (mm Hg; PDE5i/placebo) | - | 43082 | - | - | 22/21.9 | 21/20 | - | 19.9/20.8 | - | 18/19 | 18/19 | - |
| TPG (mm Hg; PDE5i/placebo) | - | 7/7 | - | - | 16.2/14.5 | 15.2/14.7 | - | 13/13 | - | 12/14 | 12/14 | - |
| DPG (mm Hg; PDE5i/placebo) | - | 2/2 | - | - | 9.6/7.8 | - | 2/-1 | - | - | - | - | |
| PVR (dyn·s/cm5; PDE5i/placebo) | - | 207/220 | - | - | 310.4/261.6 | 360/354 | - | 207/203 | - | 340/360 | 340/360 | - |
| Features of combined post- and pre-capillary PH (DPG ≥ 7 mm Hg; PVR > 3 WU (> 240 dyn·s/cm5)) | Not investigated | No | Not investigated | Not investigated | Yes | Yes | No | Mainly no (Cpc-PH in 12%) | Not investigated | Yes | Yes | Not investigated |
| Outcomes | ||||||||||||
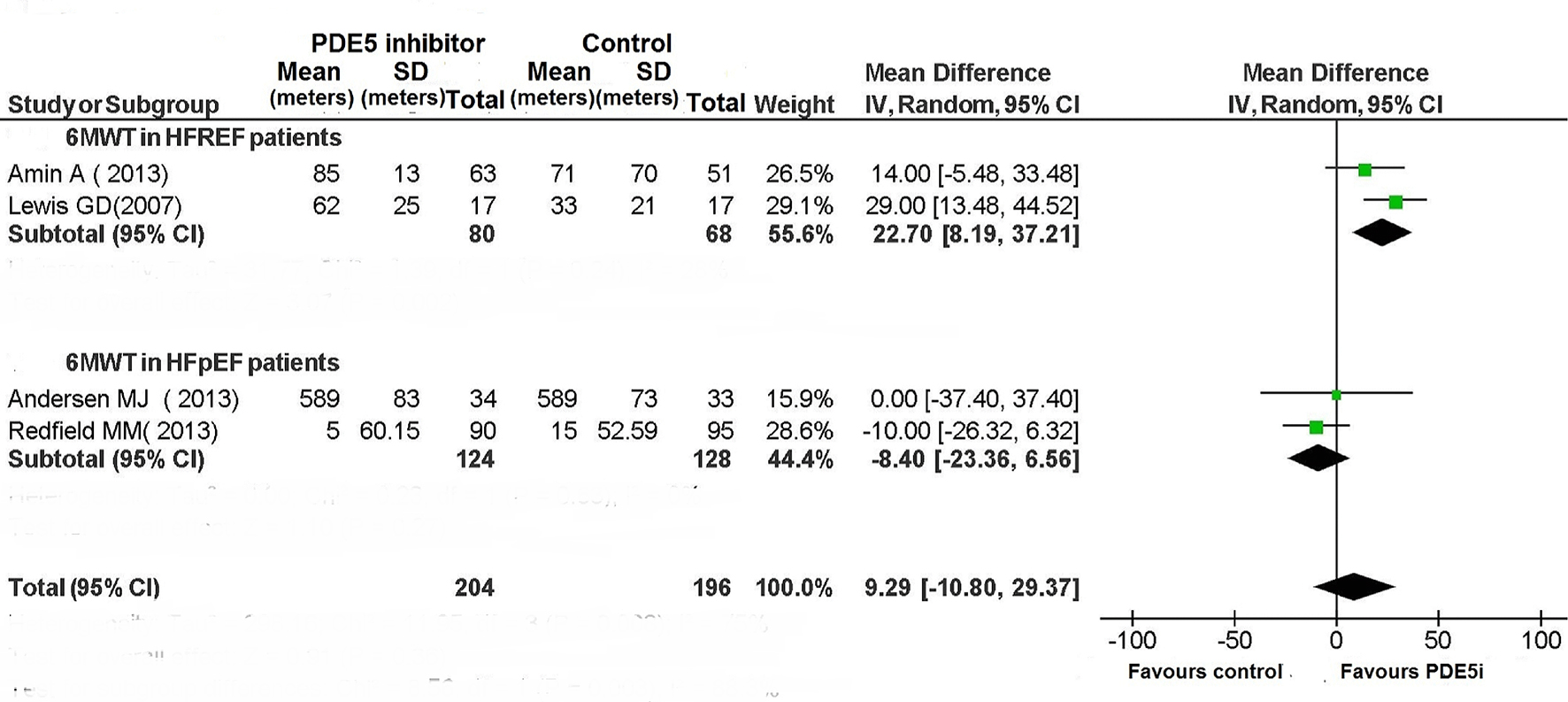
| Exercise capacity | No change | No change | Improved | Improved | N/A | Improved | Improved | No change | Improved | Improved | Improved | No change |
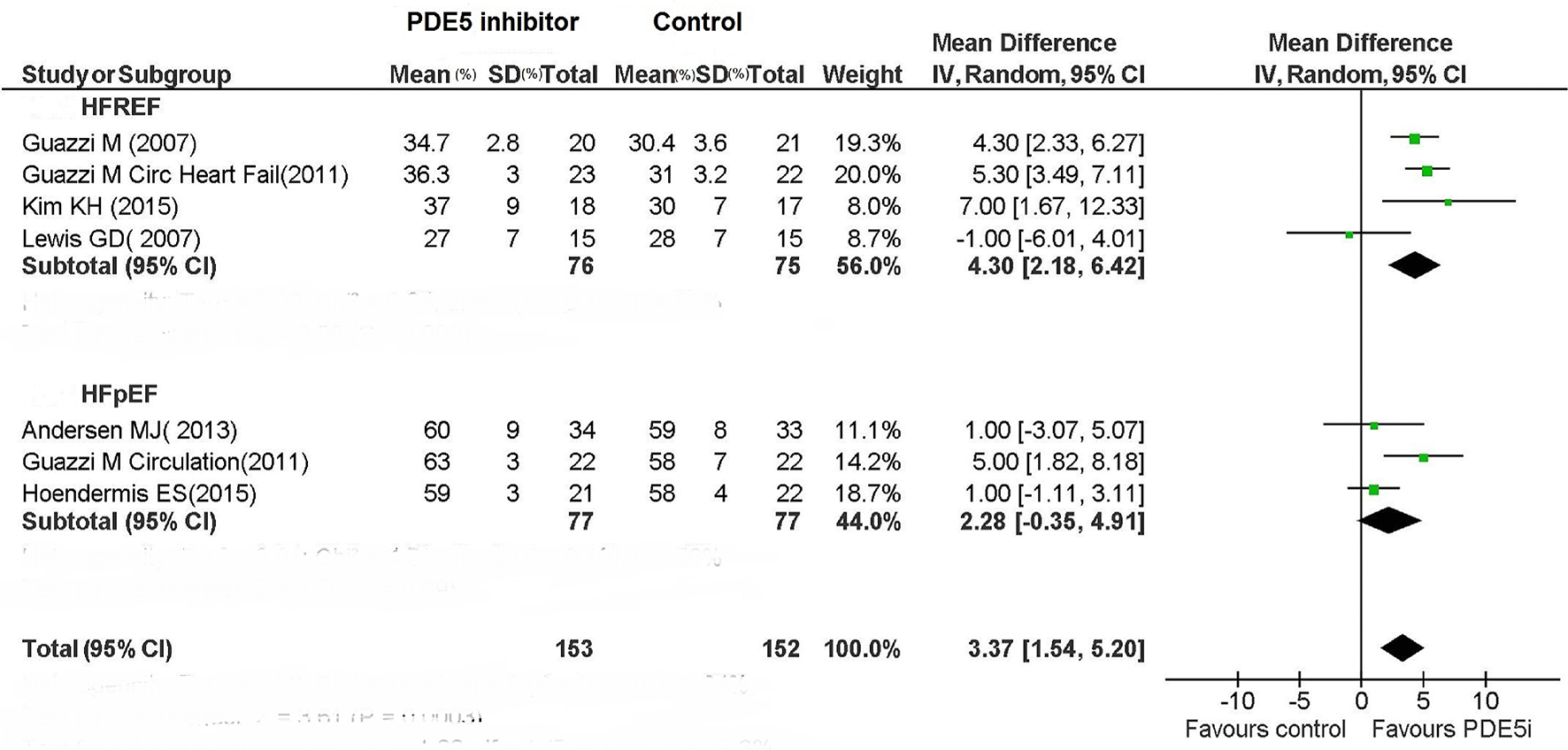
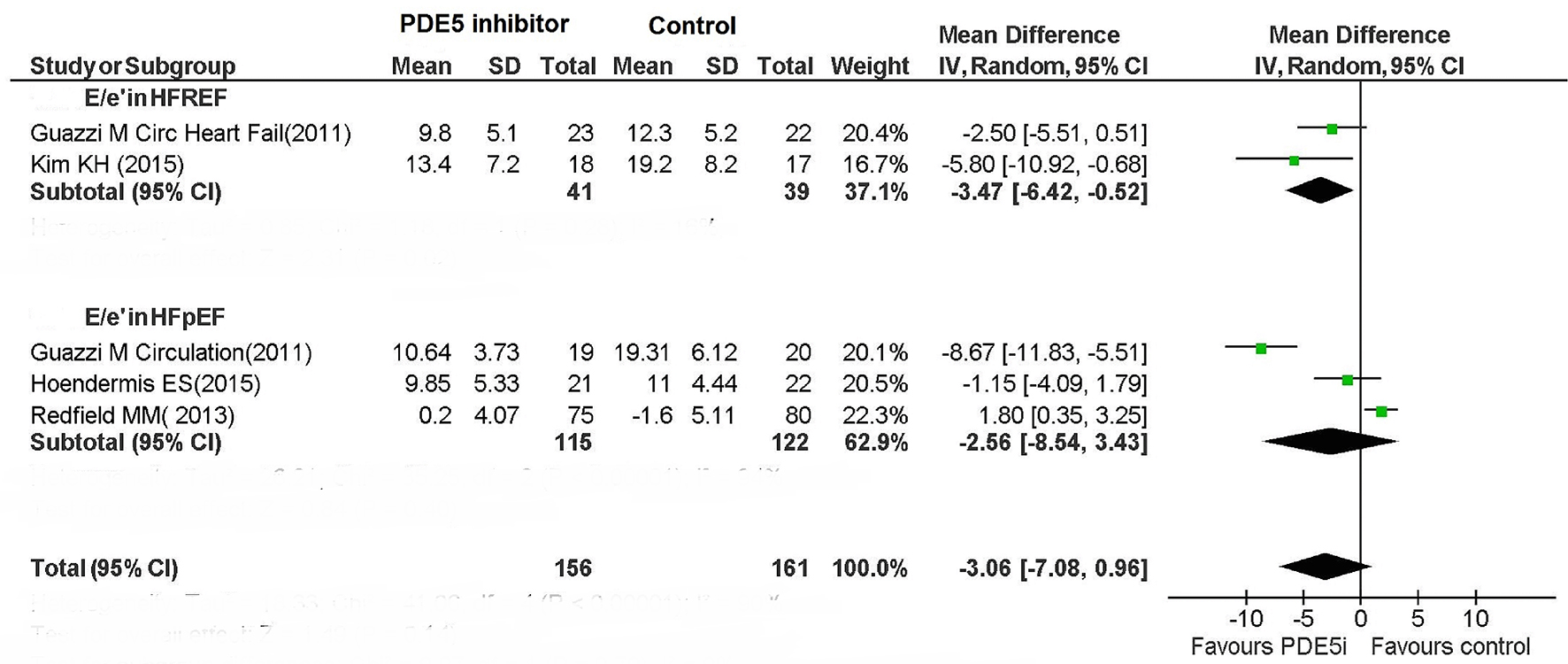
| LV function | N/A | Improved | No change | Improved | Improved | Improved | Improved | No change | Improved | N/A | Improved | No change |
| Pulmonary pressure | N/A | No change | Reduced | Reduced | Reduced | Reduced | Reduced | No change | Reduced | Reduced | Reduced | No change |