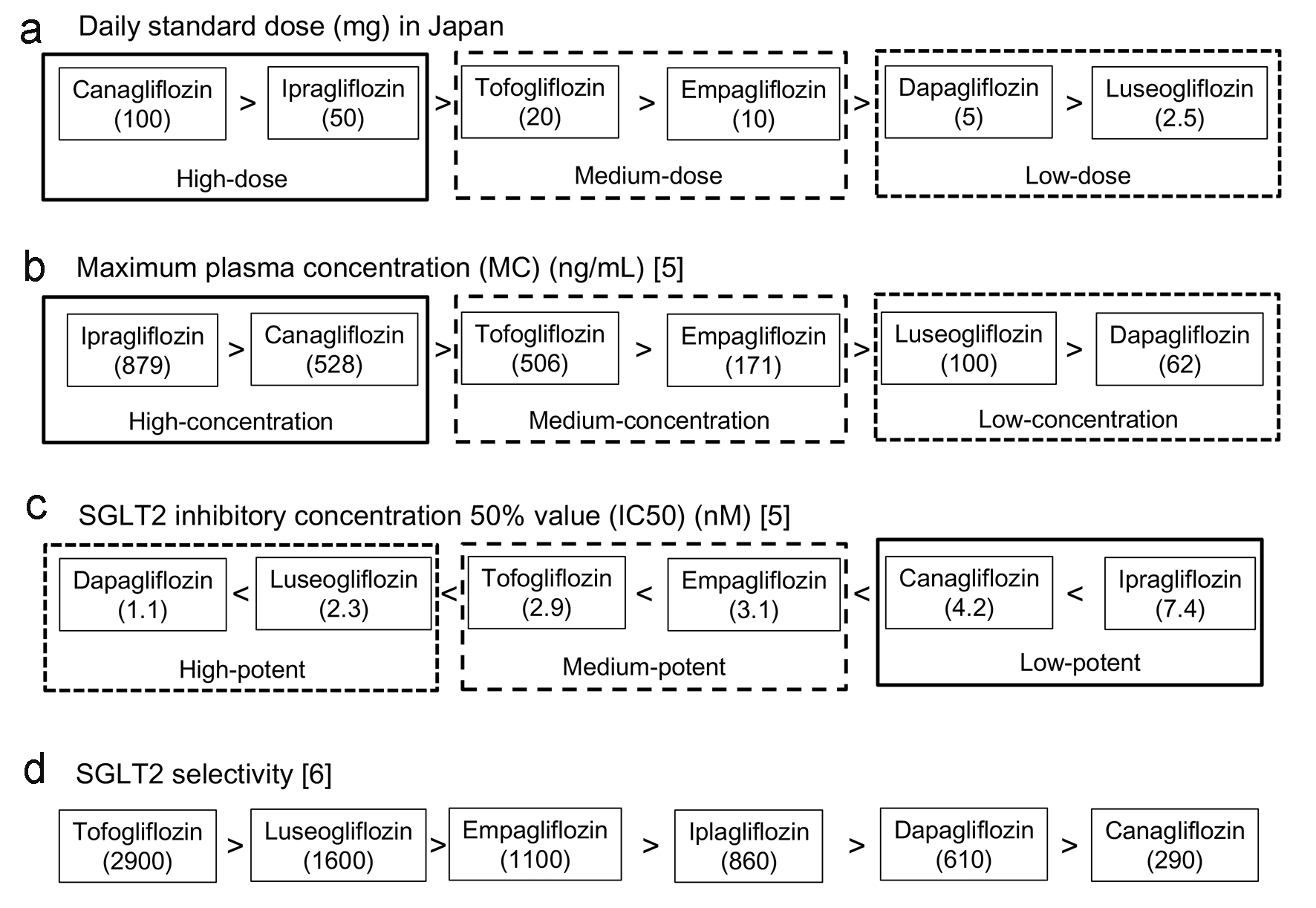
Figure 1. Daily standard dose (a), maximum plasma concentration (b), SGLT2 inhibitory concentration 50% value (c) and SGLT2 selectivity (d) of each SGLT2i. The data in (a) were obtained by the interview form of each SGLT2i produced in Japan, data in (b) and (c) were obtained from the article by Nishiyama et al [5], and data in (d) were obtained from the article by Suzuki et al [6].