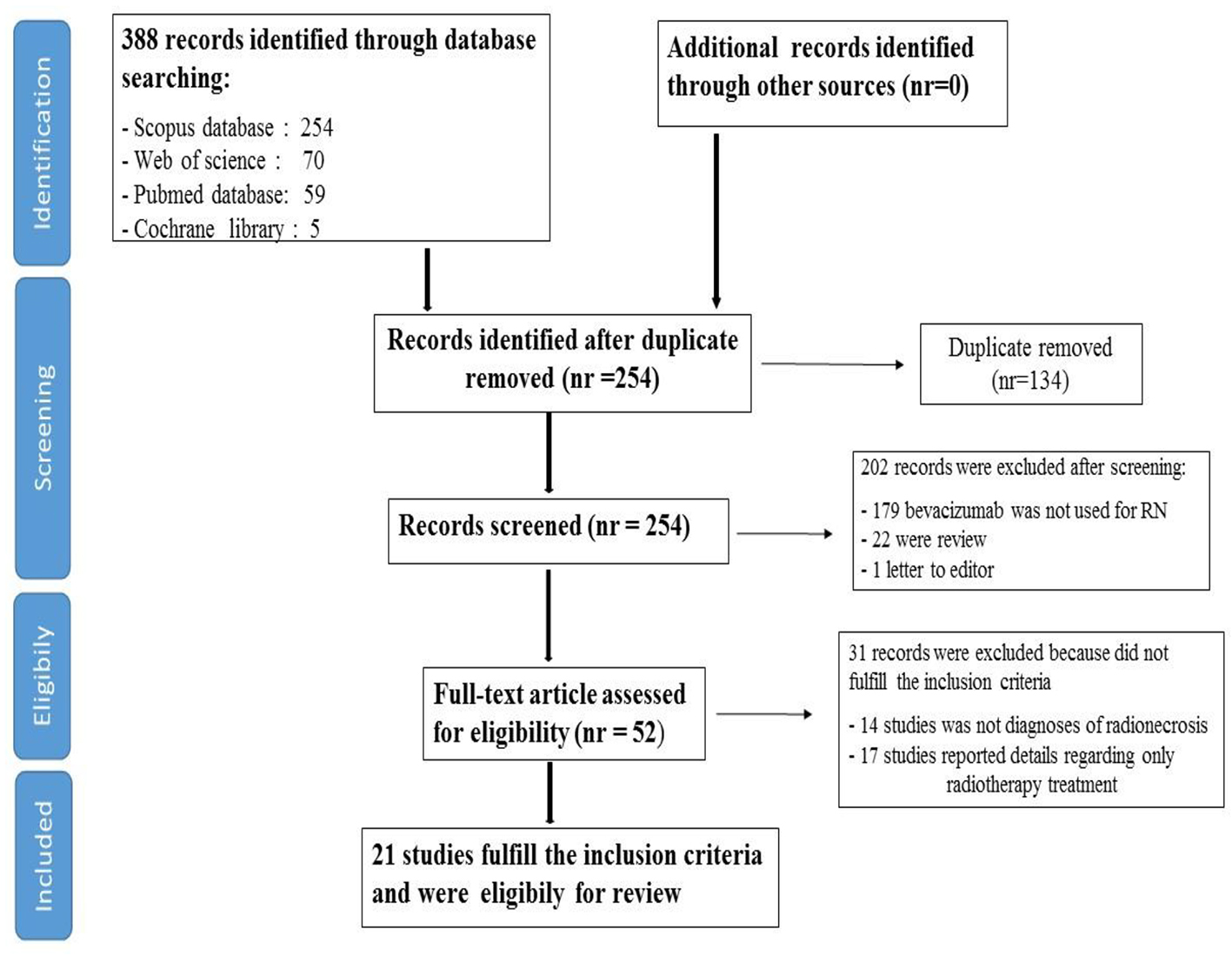
Figure 1. Flow chart of systematic literature search process.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 9, Number 4, April 2017, pages 273-280
Bevacizumab for the Treatment of Radiation-Induced Cerebral Necrosis: A Systematic Review of the Literature
Figure

Tables
| Author, year of publication | Title of the study | Institution | Journal of publication | Cases | Age | Gender |
|---|---|---|---|---|---|---|
| M: male; F: female; Dpt: department. | ||||||
| Gonzalez et al, 2007 [25] | Effect of bevacizumab on radiation necrosis of the brain | The University of Texas M. D. Anderson Cancer Center, Houston, USA | Int. J. Radiation Oncology Biol. Phys. | 8 | 54 | 4 M 4 F |
| Wong et al, 2008 [26] | Bevacizumab reverses cerebral radiation necrosis | Brain Tumor Center & Neuro-Oncology Unit, Deaconess Medical Center, Boston, USA | J Clin Oncol. | 1 | 43 | F |
| Liu et al, 2009 [31] | Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas | Dpt of Radiation Oncology, University of Colorado Denver, Aurora, USA | Int. J. Radiation Oncology Biol. Phys. | 3 | - | 1 F 2 M |
| Torcuator et al, 2009 [27] | Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis | Dpt of Neurosurgery, Henry Ford Hospital, Detroit, USA | J Neurooncol. | 6 | 48 | 3 F 3 M |
| Jeyaretna et al, 2011 [42] | Exacerbation of cerebral radiation necrosis by bevacizumab | Massachusetts General Hospital, Boston, USA | J Clin Oncol. | 1 | 35 | M |
| Benoit et al, 2011 [30] | Favorable outcome with bevacizumab after poor outcome with steroids in a patient with temporal lobe and brainstem radiation necrosis | Hospices Civils de Lyon, Neurologie, Lyon, France | J Neurol. | 1 | 38 | F |
| Matuschek et al, 2011 [33] | Bevacizumab as a treatment option for radiation-induced cerebral necrosis | Dpt of Radiation Oncology, University Hospital Dusseldorf, Dusseldorf, Germany | Strahlenther. Onkol. | 1 | 19 | M |
| Levin et al, 2011 [35] | Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the CNS | Dpt of Neuro-Oncology, University of Texas M. D, Houston, Texas, USA | Int. J. Radiation Oncology Biol. Phys. | 7 | 47 | 4 M 3 F |
| Nonoguchi et al, 2011 [12] | The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles | Department of Neurosurgery, Osaka Medical College, Takatsuki, Japan | J. Neurooncol. | 6 | 58 | 2 M 4 F |
| Sanborn et al, 2011 [32] | Treatment of steroid refractory, Gamma Knife related radiation necrosis with bevacizumab: case report and review of the literature | University of Pennsylvania, Dpt of Neurosurgery, United States | Clinical Neurology and Neurosurgery | 1 | 38 | M |
| Arratibel-Echarren et al, 2011 [28] | Use of bevacizumab for neurological complications during initial treatment of malignant gliomas | Dpt of Neuro-Oncology, University of Pennsylvania, Philadelphia, USA | Neurologia | 4 | 42 | 4 M |
| DeSalvo et al, 2012 [29] | Radiation necrosis of the pons after radiotherapy for nasopharyngeal carcinoma: diagnosis and treatment | Harvard Medical School, Boston, USA | Radiology Case | 1 | 57 | M |
| Wang et al, 2012 [34] | Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients | Dpt of Radiation Oncology, Huashan Hospital, Fudan University, Shanghai, China | European Journal of Medical Research | 17 | 48 | 13 M 4 F |
| Furuse et al, 2013 [38] | Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET | Dpt of Neurosurgery, Osaka Medical College, Takatsuki, Osaka, Japan | Jpn J Clin Oncol | 11 | 57 | 6 M 5 F |
| Boothe et al, 2013 [36] | Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery | Dpt of Radiology and Brain Tumor, Memorial Sloan-Kettering Cancer Center, New York, USA | Neuro-Oncology | 11 | 58 | 4 M 7 F |
| Alessandretti et al, 2013 [40] | Low-dose bevacizumab is effective in radiation-induced necrosis | Dpt of Oncology, Hospital Sao Jose, Sao Paulo, Brazil | Case report in Oncology | 2 | 49 | F |
| Bostrom et al, 2014 [37] | Bevacizumab treatment in malignant meningioma with additional radiation necrosis | University of Bonn Medical Center, Bonn | Strahlenther. Onkol. | 1 | 80 | F |
| Delishaj et al, 2015 [39] | The effectiveness of bevacizumab in radionecrosis after radiosurgery of a single brain metastasis | Dpt of Radiotherapy, University Hospital of Pisa, Italy | Rare Tumor | 1 | 73 | F |
| Sadraei et al, 2015 [41] | Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland Clinic experience | Brain Tumor Neuro-Oncology Center, Radiation Oncology and Solid Tumor, Cleveland | American Journal of Clinical Oncology | 24 | 57 | 9 M 15 F |
| Zhuang et al, 2015 [44] | Exploration of the recurrence in radiation brain necrosis after bevacizumab discontinuation | Dpt of Radiotherapy, Tianjin Medical University Cancer, Tianjin, China | Oncotarget. | 14 | 56 | 6 M 8 F |
| Xiang et al, 2015 [43] | Bevacizumab alleviates radiation-induced brain necrosis: a report of four cases | Dpt of Oncology, Renmin Hospital of Wuhan University, Wuhan, China | J. Cancer Res. Ther. | 4 | 58 | 3 F 1 M |
| Total number of cases included in the review | 125 | 63 M 62 F | ||||
| Author, year of publication | Bevacizumab treatment | No. of cycles (median) | Follow-up (months, median) | RN T1 post-contrast reduction (mean) | RN FLAIR reduction (mean) |
|---|---|---|---|---|---|
| Gonzalez et al, 2017 [25] | 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks | 2 - 4 | 8 | 51% | 66 |
| Wong et al, 2008 [26] | 5 mg/kg every 2 weeks | 4 | 6 | - | - |
| Liu et al, 2009 [31] | 10 mg/kg every 2 weeks | 4 | 7 | - | - |
| Torcuator et al, 2009 [27] | 10 mg/kg every 2 weeks | 2 - 4 | 6 | 79% | 49% |
| Jeyaretna et al, 2011 [42] | 5 mg/kg every 2 weeks | 4 | 5 | - | - |
| Benoit et al, 2011 [30] | 5 mg/kg every 2 weeks | 4 | 12 | - | - |
| Matuschek et al, 2011 [33] | 10 mg/kg every 2 weeks | 6 | 5 | 100% | |
| Levin et al, 2011 [35] | 7.5 mg/kg every 2 - 3 weeks | 4 | 10 | 63% | 59% |
| Nonoguchi et al, 2011 [12] | - | - | - | - | - |
| Sanborn et al, 2011 [32] | 10 mg/kg every 2 weeks | - | 4 | - | - |
| Arratibel-Echarren et al, 2011 [28] | - | - | - | ||
| DeSalvo et al, 2012 [29] | 10 mg/kg every 2 weeks | - | - | - | - |
| Furuse et al, 2013 [38] | 5 mg/kg every 2 weeks | 3 | 14.4 | 65% | 65.5% |
| Wang et al, 2012 [34] | 7.5 mg/kg every 2 weeks | 2 - 6 (4) | 6 | 54.9% | 48.4% |
| Furuse et al, 2013 [38] | 5 mg/kg every 2 weeks | 3 | 14.4 | 65% | 65.5% |
| Boothe et al, 2013 [36] | 10 mg/kg every 2 weeks | 6 | - | 56.7% | 52.7% |
| Alessandretti et al, 2013 [40] | 5 mg/kg every 2 weeks 7.5 mg/kg every 4 weeks | - | 10 | - | - |
| Bostrom et al, 2014 [37] | 5 mg/kg every 2 weeks | 4 | 4 | - | - |
| Delishaj et al, 2015 [39] | 7.5 mg/kg every 2 weeks | 4 | 8 | - | - |
| Sadraei et al, 2015 [41] | 11% 10 mg every 2 weeks or 15 mg/kg every 3 weeks 13% 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 week | 6 | 8 | 48.1% | 53.7% |
| Zhuang et al, 2015 [44] | 5 mg/kg every 3 - 4 weeks | 3 | 12 | 64.2% | - |
| Xiang et al, 2015 [43] | 7.5 mg/kg every 3 weeks | 2 | - | - | - |