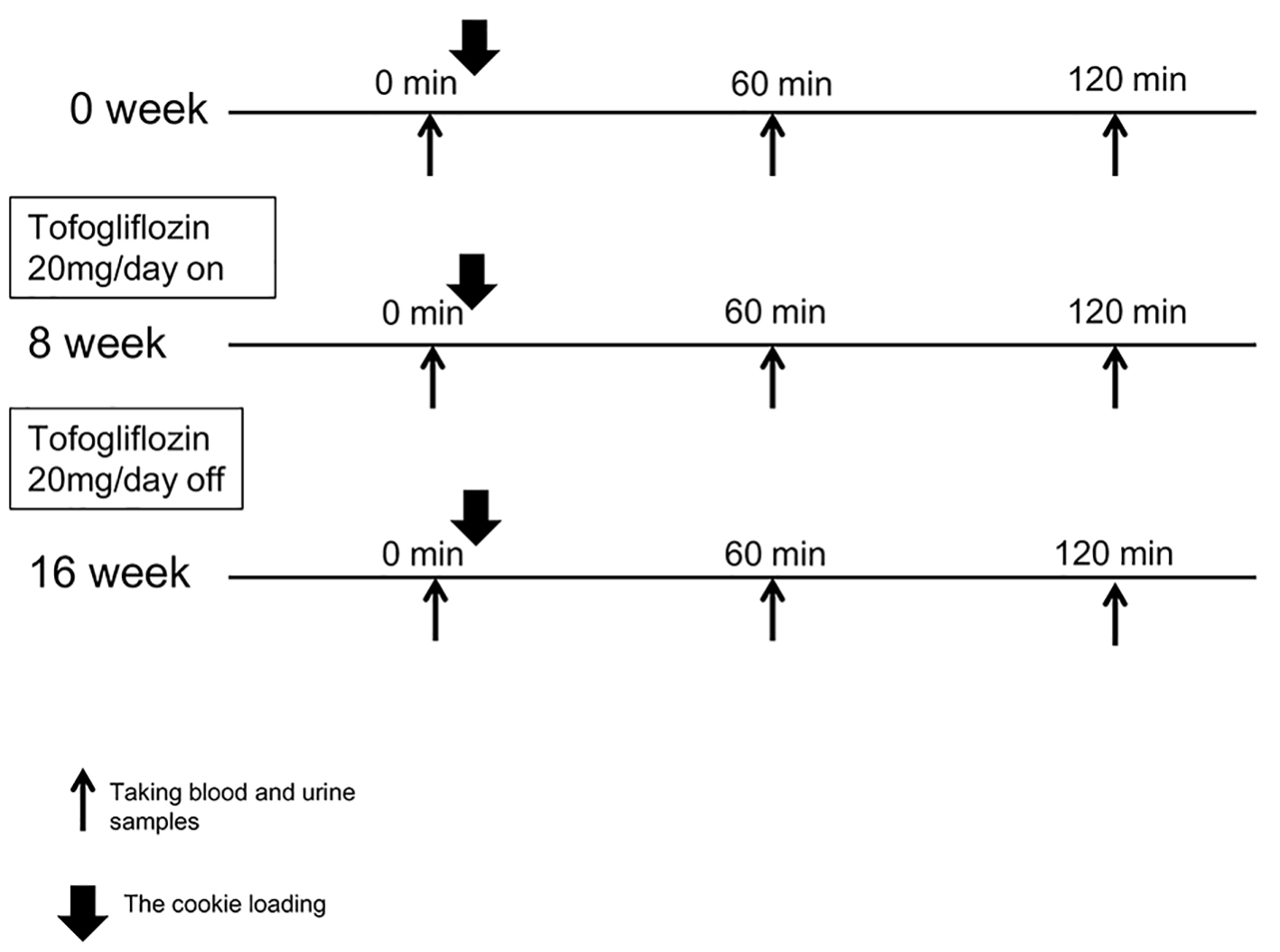
Figure 1. The study protocol is shown. We conducted the cookies tolerance test on three points: 0 week (baseline), 8 weeks (after treatment) and 16 weeks (after washout). Tofogliflozin (20 mg per day) administration started from the next day of the first cookies tolerance test and continued for 8 weeks. After 8 weeks of tofogliflozin administration and a subsequent 8-week washout of the agent, the cookies tolerance test was again conducted exactly the same way it was done before starting tofogliflozin.