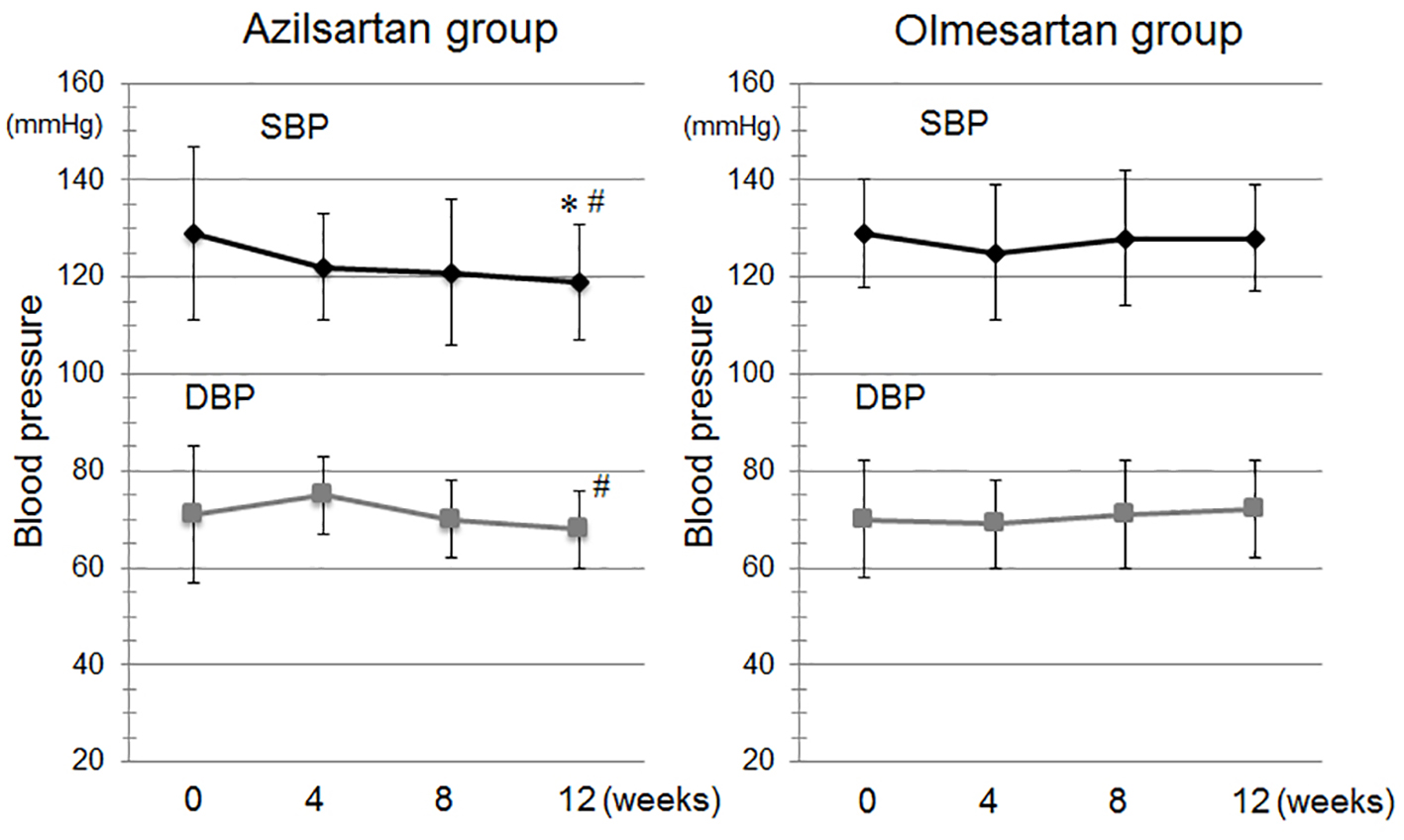
Figure 1. Time course of systolic blood pressure (SBP) and diastolic BP (DBP) during the study period in the azilsartan and olmesartan groups. *P < 0.05 vs. at baseline in the azilsartan group. #P < 0.05 vs. at 12 weeks in the olmesartan group.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 10, October 2016, pages 743-748
Depressor and Anti-Inflammatory Effects of Angiotensin II Receptor Blockers in Metabolic and/or Hypertensive Patients With Coronary Artery Disease: A Randomized, Prospective Study (DIAMOND Study)
Figures

Tables
| Azilsartan group (n = 18) | Olmesartan group (n = 20) | P value (azilsartan vs. olmesartan) | |
|---|---|---|---|
| BMI: body mass index; WC: waist circumference; MetS: metabolic syndrome; DM: diabetes mellitus; DL: dyslipidemia; HU: hyperuricemia; SBP: systolic blood pressure; DBP: diastolic BP; PR: pulse rate; LVEF: left ventricular ejection fraction; CCB: calcium channel blocker; SU: sulfonylurea; DPP4-I: dipeptidyl peptidase-4 inhibitor. | |||
| Age (years) | 68 ± 7 | 71 ± 9 | 0.24 |
| Male (%) | 78 | 65 | 0.40 |
| BMI (kg/m2) | 26.1 ± 2.7 | 26.0 ± 3.7 | 0.98 |
| WC (cm) | 94 ± 7 | 90 ± 11 | 0.18 |
| MetS (%) | 89 | 65 | 0.09 |
| Smoking (%) | 72 | 50 | 0.17 |
| DM (%) | 50 | 55 | 0.76 |
| DL (%) | 94 | 95 | 0.94 |
| HU (%) | 17 | 30 | 0.35 |
| SBP (mm Hg) | 128 ± 17 | 129 ± 11 | 0.99 |
| DBP (mm Hg) | 71 ± 14 | 70 ± 12 | 0.93 |
| PR (/min) | 67 ± 10 | 72 ± 11 | 0.18 |
| LVEF (%) | 63 ± 11 | 62 ± 9 | 0.88 |
| Medication | |||
| β-blocker (%) | 44 | 20 | 0.11 |
| α-blocker (%) | 28 | 10 | 0.17 |
| CCB (%) | 50 | 70 | 0.22 |
| Diuretics (%) | 0 | 20 | 0.046 |
| Statin (%) | 89 | 80 | 0.47 |
| Nicorandil (%) | 22 | 10 | 0.32 |
| SU (%) | 6 | 5 | 0.94 |
| Metformin (%) | 6 | 20 | 0.20 |
| DPP4-I (%) | 39 | 40 | 0.95 |
| Insulin (%) | 22 | 15 | 0.35 |
| Azilsartan group (n = 18) | Olmesartan group (n = 20) | |||||
|---|---|---|---|---|---|---|
| 0 weeks | 12 weeks | P value (0 vs. 12 weeks) | 0 weeks | 12 weeks | P value (0 vs. 12 weeks) | |
| UN: urea nitrogen; Cr: creatinine; eGFR: estimated glomerular filtration rate; UA: uric acid; Na: sodium; K: potassium; Cl: chloride; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglycerides; TC: total cholesterol; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; CK: creatinine kinase; Glu: glucose; HbA1c: hemoglobin A1c; NT-proBNP: N-terminal pro-brain natriuretic peptide. | ||||||
| UN, mg/dL | 17 ± 5 | 18 ± 5 | 0.38 | 19 ± 6 | 20 ± 9 | 0.50 |
| Cr, mg/dL | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.009 | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.08 |
| eGFR, mL/min/1.73 m2 | 63 ± 13 | 58 ± 12 | 0.003 | 58 ± 17 | 55 ± 15 | 0.55 |
| UA, mg/dL | 5.5 ± 1.1 | 5.7 ± 0.8 | 0.67 | 5.2 ± 1.1 | 5.9 ± 1.6 | 0.17 |
| Na, mEq/L | 142 ± 2 | 142 ± 2 | 0.67 | 141 ± 3 | 142 ± 3 | 0.64 |
| K, mEq/L | 4.3 ± 0.4 | 4.5 ± 0.5 | 0.07 | 4.3 ± 0.4 | 4.5 ± 0.4 | 0.30 |
| Cl, mEq/L | 105 ± 3 | 106 ± 3 | 0.12 | 106 ± 4 | 106 ± 4 | 0.70 |
| LDL-C, mg/dL | 85 ± 27 | 87 ± 38 | 0.70 | 76 ± 28 | 77 ± 23 | 0.86 |
| HDL-C, mg/dL | 47 ± 15 | 47 ± 12 | 0.80 | 43 ± 13 | 45 ± 15 | 0.10 |
| TG, mg/dL | 150 ± 71 | 144 ± 58 | 0.70 | 144 ± 86 | 139 ± 82 | 0.69 |
| TC, mg/dL | 160 ± 23 | 166 ± 42 | 0.55 | 146 ± 33 | 148 ± 30 | 0.64 |
| AST, IU/L | 27 ± 7 | 27 ± 7 | 0.96 | 27 ± 14 | 26 ± 12 | 0.60 |
| ALT, IU/L | 26 ± 9 | 27 ± 12 | 0.72 | 26 ± 14 | 24 ± 10 | 0.15 |
| LDH, IU/L | 202 ± 36 | 195 ± 28 | 0.39 | 197 ± 35 | 186 ± 31 | 0.08 |
| CK, IU/L | 132 ± 79 | 128 ± 58 | 0.80 | 117 ± 73 | 116 ± 68 | 0.97 |
| Glu, mg/dL | 115 ± 30 | 124 ± 60 | 0.56 | 121 ± 30 | 126 ± 38 | 0.51 |
| HbA1c, % | 6.6 ± 1.0 | 6.8 ± 1.3 | 0.22 | 7.0 ± 1.7 | 6.7 ± 1.5 | 0.36 |
| NT-proBNP, pg/mL | 114 ± 96 | 111 ± 104 | 0.90 | 324 ± 504 | 203 ± 258 | 0.07 |