Figure 1. Relapse for salvage autoHCT2 versus alloHCT2, P = 0.605.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 5, Number 3, June 2013, pages 174-184
Outcomes of Salvage Autologous Versus Allogeneic Hematopoietic Cell Transplantation for Relapsed Multiple Myeloma After Initial Autologous Hematopoietic Cell Transplantation
Figure

Tables
| Variable | AutoHCT2 N = 27 | AlloHCT2 N = 19 |
|---|---|---|
| * statistically significant. | ||
| Male/Female | 16/11 | 10/9 |
| Median age years (range)* | 62 (32 - 69) | 54 (43 - 63) |
| Median months from diagnosis to autoHCT1 | 8 (3 - 39) | 8 (5 - 30) |
| KPS at HCT2: ≥ 70% vs. <70% * | 20/7 | 19/0 |
| HCT comorbidity index | ||
| 0, 1 | 5 | 8 |
| 2, 3 | 10 | 7 |
| > 3 | 12 | 4 |
| Durie-Salmon stage :I/II/III/unknown | 4/6/17 | 0/5/13/1 |
| ISS stage: I/II/III/unknown | 11/4/5/7 | 9/5/3/2 |
| B2microglobulin at HCT2: ≥ 3.5/<3.5/unknown | 9/14/4 | 4/14/1 |
| Cytogenetics | ||
| High risk/intermediate | 9 | 4 |
| Standard risk | 15 | 13 |
| Unknown | 3 | 2 |
| IG subtype | ||
| IgG | 12 | 10 |
| IgA | 7 | 8 |
| Light chain | 8 | 0 |
| Nonsecretory | 1 | |
| Lines of chemo before HCT2 | 1 (1 - 5) | 2 (1 - 5) |
| Chemosensitive before HCT2:Yes/No | 11/16 | 11/8 |
| Induction before HCT2* | ||
| Conventional chemo | 6 | 12 |
| Novel agents | 21 | 7 |
| Time from autoHCT1 to relapse: months (range) | 16.5 (4 - 42) | 12 (2 - 45) |
| Time from autoHCT1 to HCT2 months (range) | 30 (5 - 104) | 21 (7 - 91) |
| conditioning for autoHCT1 | 2/7/18 | 1/12/6 |
| BuCY/BuCyVP16/melphalan | ||
| Conditioning for alloHCT2: Reduced intensity (FLU/BU N = 12, FLU/MEL N = 3, FLU/CY/TBI N = 1), Myeloablative (BU/CY N = 3) | Reduced intensity conditioning 16 | |
| Myeloablative 3 | ||
| Conditioning for autoHCT2 | 9/2/16 | |
| BuCy/ BuCyVP16/melphalan | ||
| Donor type | ||
| Matched sibling related 6/6 | 13 | |
| Matched unrelated 10/10 | 5 | |
| Haploidentical related 7/14 | 1 | |
| Stem cell type BM/PB | 0/27 | 1/18 |
| Year of HCT2* | ||
| 1995 - 2005 | 6 | 8 |
| 2006 - 2011 | 21 | 11 |
| Disease status before/after HCT2 | ||
| CR | 0/4 | 2/3 |
| VGPR | 2/11 | 3/4 |
| PR | 9/7 | 6/4 |
| SD | 1/2 | 8/0 |
| PD | 15/3 | 0/8 |
| Maintenance after HCT2: yes vs. no | 12/15 | 3/16 |
| Median months of follow up from diagnosis (range) | 57 (19 - 115) | 57 (22 - 154) |
| Variable | Allohct2 N = 19 |
|---|---|
| Donor/recipient gender | |
| M/M | 6 |
| M/F | 8 |
| F/F | 2 |
| F/M | 3 |
| Conditioning for alloHCT2 | |
| Reduced intensity conditioning | |
| FLU/BU | 12 |
| FLU/MEL | 3 |
| FLU/CY/TBI | 1 |
| Myeloablative BU/CY | 3 |
| GVHD prophylaxis | |
| FK | 11 |
| FK/MTX | 7 |
| CSA/MMF | 1 |
| DLI use, yes/no | 10/9 |
| ATG use, yes/no | 15/4 |
| Acute GVHD | |
| None | 6 |
| I-II | 6 |
| III-IV | 7 |
| Chronic GVHD | |
| None | 12 |
| Limited | 2 |
| Extensive | 5 |
| Causes of death | |
| PD | 5 |
| GVHD | 3 |
| Infection | 3 |
| Renal failure | 1 |
| Variable | Qazilbash et al | This study |
|---|---|---|
| Year inclusive | 1992 - 2006 | 1995 - 2011 |
| # of patients autoHCT2/alloHCT2 | 14/26 | 27/19 |
| Time from AutoHCT1 and AutoHCT2 (months) | 25 | 30 |
| Time from AutoHCT1 to AlloHCT2 (months) | 17 | 21 |
| Disease response post autoHCT2, CR/VGPR/PR | 21%/-/43% | 15%/41%/26% |
| Disease response post alloHCT2, CR/VGPR/PR | 31%/-/38% | 16%/21%/21% |
| NRM after autoHCT2/alloHCT2 | 7%/11% | 3.7%/5.3% |
| Median PFS post autoHCT2/alloHCT2 (months) | 6.8/7.3 | 19/6 |
| Median OS post autoHCT2/alloHCT2 (months) | 29.5/13 | 23/19 |
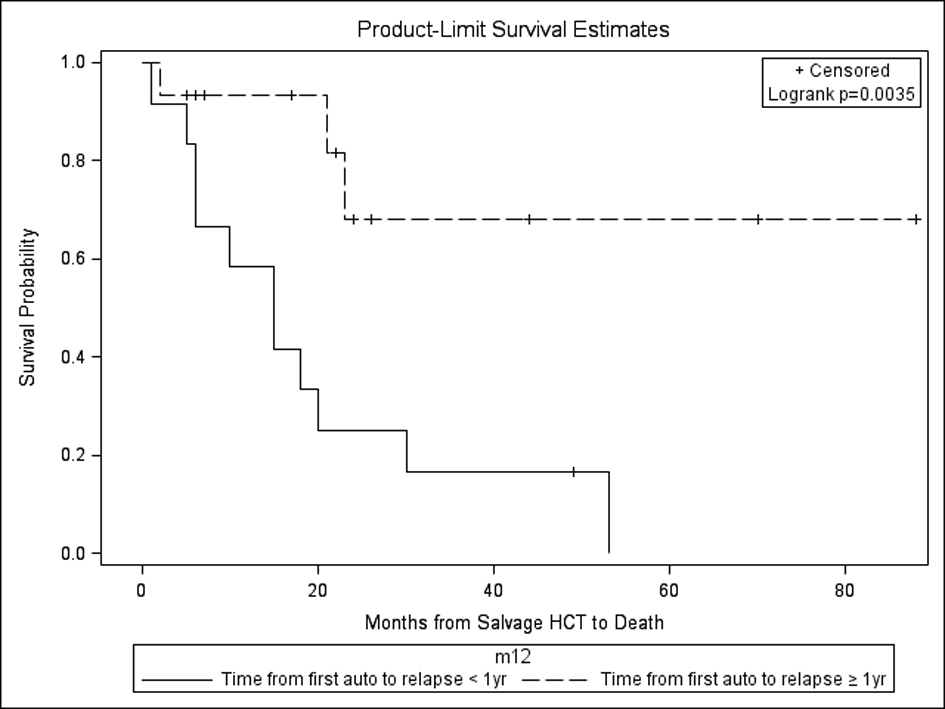
| Prognostic Factors | Univariate analysis: time from autoHCT1 to alloHCT2 > 1 year (P = 0.02) predicted significantly better OS for alloHCT2 No factors impacted OS in autoHCT2 group | Multivariate analysis: relapse from autoHCT1 ≥ 1 year favorably impacted PFS and OS after autoHCT2. Also, maintenance therapy after autoHCT2 favorably impacted OS after autoHCT2. No factors impacted PFS/OS after alloHCT2. |