|
|||||
|
|
|||||
| Review | |||||
|
|
|||||
| Volume 2, Number 1, February 2010, Pages 1-17 | |||||
|
|
|||||
|
Na+, K+-ATPase: Ubiquitous Multifunctional Transmembrane Protein
and its Relevance
to Various Pathophysiological Conditions Mohd Suhaila, b
aDepartment of
Biochemistry, University of Allahabad, Allahabad-211002, India
Manuscript accepted for publication February 5, 2010
Abstract
The
Na+, K+-ATPase (NKA)
is an
ubiquitous
enzyme
consisting of α,
β
and γ
subunits, and is responsible for the creation and maintenance of the
Na+
and K+
gradients
across the cell membrane by transporting 3
Na+
out and 2 K+ into
the cell.
Sodium pump regulation is tissue
as well as isoform specific. Intracellular messengers differentially
regulate the activity of the individual NKA isozymes. Regulation of
specific NKA isozymes gives cells the ability to precisely
coordinate NKA activity to their physiological requirements. It is
the only known receptor for the cardiac glycosides used to treat
congestive heart failure and cardiac arrhythmias. Endogenous ligands
structurally similar to cardiac glycosides may act as natural
regulators of the sodium pump in heart and other tissues.
Identification of naturally occurring regulators of NKA could
initiate the discovery of new hormone-like control systems involved
in the etiology of selected disease processes, hence the importance
of understanding the relation of the sodium pump and its ligands to
disease. Diabetes has a marked effect on the metabolism of
a variety of tissues and because the NKA is critical for the
membrane potential and many transports, a change in its activity in
diabetes would have profound consequence in these tissues. NKA is
also involved in hypertension, salt balance, cardiovascular and
renal disorders, sperm capacitation, cell volume regulation,
apoptosis, rheumatoid arthritis, sepsis, neurological disorders,
lung edema clearance and preeclampsia. NKA activity and expression
in the collecting duct of kidney are modulated physiologically by
hormones like aldosterone, vasopressin, and insulin. NKA enzyme
activity and subunit levels are reduced in carcinoma,
NKA-β
levels were highly reduced in an invasive form of human renal clear
cell carcinoma, androgen-dependent prostate cancer, in early stages
of urothelial cancer, as well as in poorly differentiated, highly
motile carcinoma cell lines obtained from various tissues suggesting
a functional link between reduced NKA-b
expression and cancer progression. It could be a target for the
development of anticancer drugs as it serves as a signal transducer,
it is a player in cell adhesion and its aberrant expression and
activity are implicated in the development and progression of
different cancers. keywords: Na+, K+-ATPase (NKA); Cardiotonic steroids (CTS); Diabetes; Hypertension; Cardiovascular and renal disorders; Signal transducer; Anticancer drugs
Introduction The transport ATPases were reported in 1957 by a Danish scientist named J.C. Skou [2]. This was the first report suggesting that Na+ and K+ transport across the plasma membrane is linked to activation of NKA (otherwise known as sodium pump). Forty years later, in 1997, J.C. Skou shared the Nobel Prize in Chemistry for his discovery of NKA. Transport ATPases have been classified into three categories: (1) P-type ATPases that catalyze reactions using a phosphorylated intermediate; (2) V-type ATPases that are found to be associated with vacuoles; and (3) F-type ATPases that are also known as ATP synthases. The primary role of the P-type NKA is to maintain the homeostasis of Na+ and K+ ions in eukaryotic cells. The purpose of this review is to highlight the structure of NKA and the relevant literature information showing its involvement in various patho-physiological processes, which may help others for further exploration to control diseases where NKA is involved.
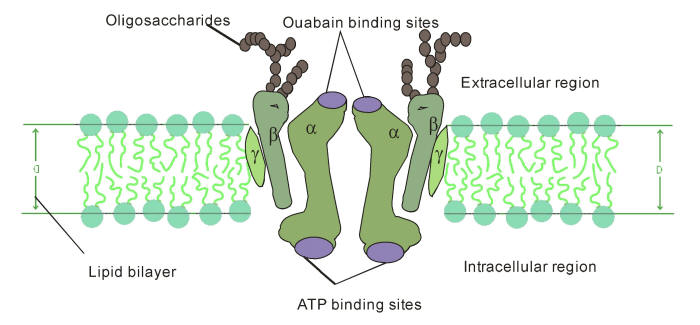
The NKA contains 1 principal catalytic subunit,
designated
α and 1 sugar-rich
auxiliary subunit, designated
β.
There is an associated subunit γ present only in some
tissues. The α-subunit has a molecular mass of about 110 kDa with 10
transmembrane segments. Its four distinct isoforms have been
identified. The differences of amino acid sequences among the
isoforms are minor. They are each coded by a different gene, some of
them located on different chromosomes [3, 4]. The various isoforms
differ primarily in their tissue distribution, α1 predominating in
several tissues, including kidney, nerves, and lung;
α2 in skeletal muscle and heart; α3 in the brain; and α4,
which is apparently localized to testis and specifically to
spermatozoa [5].
The α-subunit
carries the catalytic function of the enzyme, and this is reflected
in its possession of several binding and functional domains. The
β-subunit has a molecular
weight of about 55 kDa, with a single membrane crossing. Its three
isoforms have been identified. As
α
isoforms,
β
isoforms have a tissue-specific distribution,
β1 is ubiquitous, β2 is
expressed in skeletal muscle and heart, and β3 in testis and central
nervous system [6]. It is clear that an essential role for
β subunit is in the
delivery and the appropriate insertion of α subunit in the
membrane [7]. In recent years, a variety of studies suggest that the
β subunit may be more
intimately involved in the mechanism of active transport and may be
a regulatory subunit [5, 8]. The
γ subunit is a
hydrophobic and a single-membrane crossing protein of molecular
weight about 12 kDa
[Fig. 1].
Although much is not known about its
function, it does appear to be obligatorily associated with the αβ
complex [9]. Further, the other family of small membrane
proteins i.e. FXYD proteins exist widely distributed in mammalian
tissues with prominent expression in tissues that perform fluid and
solute transport or that are electrically excitable.
The FXYD protein family is a family of small membrane
proteins that share a 35-amino acid signature sequence domain,
beginning with the sequence PFXYD and containing 7 invariant and 6
highly conserved amino acids. The approved human gene nomenclature
for the family is FXYD-domain containing ion transport regulator.
Recent experimental evidence suggests that at least five of the
seven members of this family, FXYD1 (phospholemman), FXYD2
(g-subunit
of NKA), FXYD3 (Mat-8), FXYD4 (CHIF), and
FXYD7, are auxiliary subunits of NKA and regulate
NKA activity in a tissue.
These results highlight the complexity of the regulation of Na+
and K+ handling by NKA, which is necessary to ensure
appropriate tissue functions such as renal Na+
reabsorption, muscle contractility, and neuronal excitability.
Moreover, a mutation in FXYD2 has been linked to cases of human
hypomagnesemia, indicating that perturbations in the regulation of
NKA by FXYD proteins may be critically involved in
pathophysiological states [10].
In 2005, Capasso et al [14] have
reported that hypertonicity-mediated upregulation of the
γ-subunit of NKA is dependent on both the c-Jun NH2-terminal kinase
(JNK) and the phosphoinositide 3-kinase (PI3 kinase) pathways. They
explored the mechanisms whereby these pathways regulate the
expression of the γ-subunit in inner medullary collecting duct cells
(IMCD3). Inhibition of JNK with SP-600125 (20
mM,
an anthrapyrazolone inhibitor of JNK), a concentration that causes
an approximately 95% inhibition of hypertonicity-stimulated JNK
activation, markedly decreased the amount of the γ-subunit in
response to 550 mosmol/kg H2O for 48 h. This was
accompanied by a parallel decrease in the γ-subunit mRNA. The rate
at which the γ-subunit mRNA decreased was unaffected by actinomycin
D. In contrast, inhibition of PI3 kinase with LY-294002 (a selective
PI3K inhibitor) results in a marked decrease in the amount of
γ-subunit protein but without alteration in γ-subunit message. The
rate at which the γ-subunit protein decreased was unaffected by
cyclohexamide. Transfection of IMCD3 cells with a γ-subunit
construct results in the expression of both γ-subunit message and
protein. However, in cortical collecting duct cells (M1 cells) such
transfection resulted in expression of only the message and not the
protein. They concluded that JNK regulates the γ-subunit at the
transcriptional level while PI3 kinase regulates γ-subunit
expression at the translational level. There is also
post-transcriptional cell specificity in the expression of the
γ-subunit of NKA.
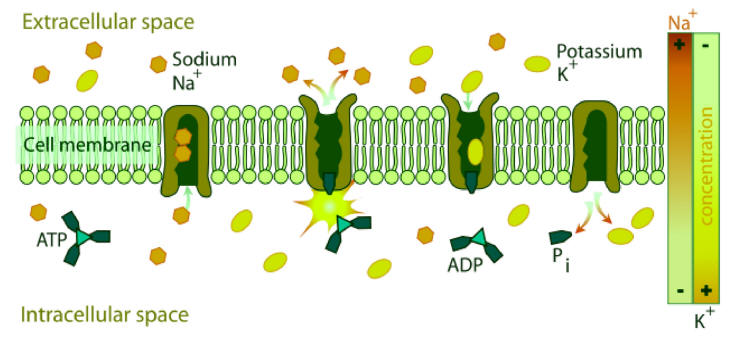
NKA couples the energy released in
the intracellular hydrolysis of ATP to the export of three
intracellular sodium ions and the import of two extracellular
potassium ions. The continuous operation of this macromolecular
machine ensures the generation and maintenance of concentration
gradients of sodium and potassium across the cell membrane
(Fig. 2). This electrochemical
gradient provides energy for the membrane transport of metabolites,
nutrients, and ions. This electrochemical gradient is essential also
for regulation of cell volume and intracellular pH and for the
action potential of muscle and nerve [9]. This enzyme is responsible
of about 15% to 20% of resting energetic expense in whole organism
[16].
Because several cellular transport systems are
coupled to the movement of sodium and, therefore, to the function of
NKA, this enzyme is the target of multiple regulatory mechanisms
activated in response to changing cellular requirements. The demand
for modulators of the NKA is likely to be greatest in tissues in
which disturbances of the intracellular alkali cation concentration
underlie their specialized functions. Prime examples are the changes
in enzyme activity that occur in response to physiological stimuli
such as nerve impulse propagation and exercise [9]. Generally,
regulation of NKA is brought about by increased message
transcription, increased recruitment of heterodimers to the cell
membrane, modifications of heterodimers trafficking, and half-life
in the cell membrane, and by direct regulation of enzymes in the
cell membrane. Direct regulation of the cell membrane enzymes
results from phosphorylation and dephosphorylation by protein
kinases and protein phosphatases. Thus, depending on the tissue,
activation of protein kinases can induce an increase or decrease in
sodium pump activity [17]. Moreover, sodium pump regulation seems to
be tissue but also isoform specific [18]. Firstly, the simplest and
most straightforward determinants of pump activity are the
concentrations of substrates- Na+, K+, and
ATP. Some hormones appear to alter NKA activity by modifying its
apparent affinity for sodium or by enhancing the sodium influx. The
ATP concentration is generally saturating for the enzyme in most
cells. However, in some tissues and under certain conditions, ATP
levels may go down to sub-saturating levels, such as in kidney
medulla, which functions under near anoxic conditions [19].
Secondly, endogenous cardiotonic steroids such as ouabain inhibits
specifically the sodium pump, whereas interactions of the pump with
components of the cytoskeleton permits the correct processing and
targeting of sodium pumps to the appropriate membrane compartment
[9]. Thirdly, NKA is a transmembranous enzyme and many reports have
focused on the role of membrane lipids. In general, lipids that
promote bilayer formation of physiological thickness and increased
fluidity tend to support optimal NKA activity, as do negatively
charged lipids such as phosphatidylserine and phosphatidylglycerol
[20-22]. The effects of cholesterol on enzyme activity are
often also related to membrane fluidity
[23]. Free fatty acids present in the membranes or as the
products of phospholipases and eicosanoids tend to have various
regulatory effects on NKA activity. Lastly, the enzyme activity is
subjected to both short- and long-term regulation by several
hormones. Short-run regulation involves generally direct effects on
the kinetic behavior of the enzyme or translocation of sodium pumps
between intracellular stores and the plasma membrane. Long-run
regulation induces de novo NKA synthesis or degradation.
Corticosteroids and particularly aldosterone sustains a long-run
increase in expression of sodium pumps, whereas catecholamines have
various affects on pump activity, with an inhibitory effect of
dopamine and a stimulating effect of epinephrine and norepinephrine
[9]. Insulin mainly stimulates the NKA activity by increasing
the translocation of sodium pumps from intracellular stores to the
cell surface, the cytoplasmic sodium content, and also the apparent
affinity of the enzyme for sodium [24]. Recently, C-peptide (a
peptide that is
made when proinsulin is split into insulin and
C-peptide;
–one C-peptide for each insulin molecule)
has been found to stimulate the NKA activity in renal tubular from
control rat [25] and in sciatic nerve from diabetic rat [26]. In
human diabetes, the decrease of availability of both insulin and
C-peptide could change the regulatory equilibrium of NKA activity in
favor of a decrease.
Isoenzymic forms of NKA
As mentioned above the NKA is characterized by a
complex molecular heterogeneity that results from the expression and
differential association of multiple isoforms of both its
α-
and
α-subunits.
At present, as many as four different
α-polypeptides
(α1,
α2,
α3,
and
α4)
and three distinct
β-isoforms
(β1,
β2,
and
β3)
have been identified in mammalian cells. The stringent constraints
on the structure of the sodium pump isozymes during evolution and
their tissues specific and developmental pattern of expression
suggests that the different NKA have evolved distinct properties to
respond to cellular requirements. The kinetic characteristics of
different NKA isozymes to the activating cations (Na+ and
K+), the substrate ATP, and the inhibitors
Ca2+ and ouabain
demonstrate that each isoform has distinct properties. In addition,
intracellular messengers differentially regulate the activity of the
individual NKA isozymes. Thus, the regulation of specific sodium
pump isozymes gives cells the ability to precisely coordinate NKA
activity to their physiological requirements [27]. Pathophysiological relevance of NKA
Alterations in NKA activity in
diabetes NKA
correlation with hypertension, impact on salt balance and vascular
contractility Although advantage has been taken of this site to treat congestive
heart failure with drugs such as digoxin, it is unknown whether this
site has a natural function in vivo. However, this site plays an
important role in the regulation of blood pressure, and it
specifically mediates adrenocorticotropic hormone (ACTH)-induced
hypertension in mice. Dostanic-Larson et al [51]
used genetically engineered mice in which the NKA
α2
isoform, which is normally sensitive to cardiac glycosides, was made
resistant to these compounds. Chronic administration of ACTH caused
hypertension in mice but not in mice with an ouabain-resistant
α2
isoform of NKA. This finding
demonstrates that the cardiac glycoside binding site of the NKA
plays an important role in blood pressure regulation, most likely by
responding to a naturally occurring ligand. Because the
α1
isoform is sensitive to cardiac glycosides in humans, they developed
mice in which the naturally occurring ouabain-resistant
a1
isoform was made ouabain-sensitive. Mice with the ouabain-sensitive
"human-like"
α1
isoform and an ouabain-resistant
α2
isoform developed ACTH-induced hypertension to greater extent than
control animals. This result indicates that the cardiac glycoside
binding site of the
α1 isoform can also mediate ACTH-induced
hypertension. Conclusively, these data provide conclusive evidence
that the cardiac glycoside binding site, which mediates the
pharmacological effects of digitalis and related drugs used in the
treatment of congestive heart failure, is also the receptor for
endogenous ligands involved in the regulation of cardiovascular
function in vivo. Such results support the hypothesis that a steroid
hormone may exist that regulates blood pressure through the
interaction with the NKA. Alterations in NKA activity in cardiovascular and renal disorders
The dysregulation of chief electrolytes
especially sodium, potassium and calcium has a
characteristic role in the cardiovascular and
renal diseases. There occurs altered sodium and potassium
concentrations in cardiovascular and renal patients primarily due to
impaired NKA activity. Alterations take place in
erythrocyte and plasma ionic environment in cardiovascular and renal
patients. Under physiological conditions, NKA pump is the
principal transporter, accounting for 1.4-2.0 mmol/rbc/hr. The Na+,
K+-cotransport and sodium leak pathway are each
responsible for approximately 0.2 mmol/rbc/hr. Most of the studies
indicate that elevation of intracellular sodium and potassium was
associated with a reduced activity of erythrocyte NKA pump [57].
Inhibition of NKA activity is the main factor as in most of the
cardiovascular diseases, inhibited or reduced ATPase activity has
been observed. The decreased serum sodium and increased serum
potassium concentrations in renal patients have also been reported
indicating that hyperkalemic state might have developed from a shift
of potassium from intracellular to extracellular compartment or it
could have been secondary to decreased renal potassium excretion.
This increased potassium could also result from decreased
renin production, which affects the aldosterone
synthesis due to adrenal defect, which then would produce renal
tubular secretory defect leading to abnormal distribution of
potassium between intra and extracellular compartments [58].
Regulatory
role of nitric oxide on NKA
in
hypertension
Oxygen-induced
regulation of NKA
|
|||||
|
|
|||||
| References | |||||
| 1. |
Skou JC,
Esmann M. The Na,K-ATPase. J Bioenerg Biomembr 1992;24(3):249-261.
[Medline] |
| 2. | Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerve. Biochim Biophys Acta 1957; 23: 394-401. |
| 3. |
Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta
1989;988(2):185-220. |
| 4. |
Mercer RW.
Structure of the Na,K-ATPase. Int Rev Cytol 1993;137C:139-168. [Medline] |
| 5. |
Kaplan JH.
Ion movements through the sodium pump. Annu Rev Physiol 1985;47:535-544.
[Medline] |
| 6. |
Kotyk A,
Amler E. Na,K-adenosinetriphosphatase: the paradigm of a membrane
transport protein. Physiol Res 1995;44(5):261-274. [Medline] |
| 7. |
McDonough
AA, Geering K, Farley RA. The sodium pump needs its beta subunit. FASEB
J 1990;4(6):1598-1605. [Medline] |
| 8. |
Geering
K. The functional role of beta subunits in oligomeric P-type ATPases. J
Bioenerg Biomembr 2001;33(5):425-438. [Medline] [CrossRef] |
| 9. |
Therien
AG, Goldshleger R, Karlish SJ, Blostein R. Tissue-specific distribution
and modulatory role of the gamma subunit of the Na,K-ATPase. J Biol Chem
1997;272(51):32628-32634. [Medline] [CrossRef] |
| 10. |
Geering
K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal
Physiol 2006;290(2):F241-250. [Medline] [CrossRef] |
| 11. |
Minor NT,
Sha Q, Nichols CG, Mercer RW. The gamma subunit of the Na,K-ATPase
induces cation channel activity. Proc Natl Acad Sci U S A
1998;95(11):6521-6525. [Medline] [CrossRef] |
| 12. |
Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit
modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J Biol Chem
1999;274(47):33183-33185. [Medline] [CrossRef] |
| 13. |
Kuster B, Shainskaya A, Pu HX, Goldshleger R, Blostein R, Mann M,
Karlish SJ. A new variant of the gamma subunit of renal Na,K-ATPase.
Identification by mass spectrometry, antibody binding, and expression in
cultured cells. J Biol Chem 2000;275(24):18441-18446. [Medline] [CrossRef] |
| 14. |
Capasso JM, Rivard CJ, Berl T. Synthesis of the Na-K-ATPase
gamma-subunit is regulated at both the transcriptional and translational
levels in IMCD3 cells. Am J Physiol Renal Physiol 2005;288(1):F76-81.
[Medline] [CrossRef] |
| 15. |
Rivard
CJ, Almeida NE, Berl T, Capasso JM. The gamma subunit of Na/K-ATPase: an
exceptional, small transmembrane protein. Front Biosci
2005;10:2604-2610. [Medline] |
| 16. |
Clausen
T, Van Hardeveld C, Everts ME. Significance of cation transport in
control of energy metabolism and thermogenesis. Physiol Rev
1991;71(3):733-774. [Medline] |
| 17. |
Ewart HS,
Klip A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms
underlying rapid and sustained changes in pump activity. Am J Physiol
1995;269(2 Pt 1):C295-311. [Medline] |
| 18. |
Feraille
E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium
transport in the kidney: hormonal control. Physiol Rev
2001;81(1):345-418. [Medline] |
| 19. |
Brezis M,
Rosen S. Hypoxia of the renal medulla--its implications for disease. N
Engl J Med 1995;332(10):647-655. [Medline] [CrossRef] |
| 20. |
Kimelberg
HK, Papahadjopoulos D. Phospholipid requirements for (Na + + K +) -ATPase
activity: head-group specificity and fatty acid fluidity. Biochim
Biophys Acta 1972;282(1):277-292. [Medline] |
| 21. |
Kimelberg HK. Alterations in phospholipid-dependent (Na+ +K+)-ATPase
activity due to lipid fluidity. Effects of cholesterol and Mg2+. Biochim
Biophys Acta 1975;413(1):143-156. [Medline] [CrossRef] |
| 22. |
Johannsson A, Smith GA, Metcalfe JC. The effect of bilayer thickness on
the activity of (Na+ + K+)-ATPase. Biochim Biophys Acta
1981;641(2):416-421. [Medline] [CrossRef] |
| 23. |
Giraud F,
Claret M, Bruckdorfer KR, Chailley B. The effects of membrane lipid
order and cholesterol on the internal and external cationic sites of the
Na+-K+ pump in erythrocytes. Biochim Biophys Acta 1981;647(2):249-258.
[Medline] [CrossRef] |
| 24. |
Sweeney
G, Klip A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol
Cell Biochem 1998;182(1-2):121-133. [Medline] [CrossRef] |
| 25. |
Ohtomo Y, Aperia A, Sahlgren B, Johansson BL, Wahren J. C-peptide
stimulates rat renal tubular Na+, K(+)-ATPase activity in synergism with
neuropeptide Y. Diabetologia 1996;39(2):199-205. [Medline] [CrossRef] |
| 26. |
Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD,
et al. Prevention of vascular and neural dysfunction in diabetic rats by
C-peptide. Science 1997;277(5325):563-566. [Medline] [CrossRef] |
| 27. |
Blanco G,
Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure,
diversity in function. Am J Physiol 1998;275(5 Pt 2):F633-650. [Medline] |
| 28. |
Gupta DK,
Ahmad F, Suhail M. Electrophoretic analysis of polypeptides and
glycopeptides of erythrocyte membrane sampled from rats simulating mild
insulin dependent diabetes mellitus. Indian J Exp Biol
1998;36(9):934-937. [Medline] |
| 29. |
Gupta DK,
Ahmad F, Suhail M. Effect of alloxan induced mild insulin dependent
diabetes mellitus on rat erythrocyte cytosolic dehydrogenases. Indian J
Exp Biol 1996;34(3):262-263. [Medline] |
| 30. | Gupta DK, Ahmad F, Suhail M. In Vivo Alloxan Induced Alterations in the Rat Erythrocyte Membrane Bound Adenosine Triphosphatases. Bioved 1991; 2:141-146. |
| 31. |
Suhail M,
Rizvi SI. Regulation of red cell acetylcholinesterase activity in
diabetes mellitus. Indian J Exp Biol 1990;28(3):234-236. [Medline] |
| 32. |
Suhail M,
Rizvi SI. Erythrocyte membrane acetylcholinesterase in type 1
(insulin-dependent) diabetes mellitus. Biochem J 1989;259(3):897-899.
[Medline] |
| 33. |
Suhail M, Rizvi S. Effect of type I
(insulin-dependent) diabetes mellitus on key glycolytic enzymes of red
blood cells. Acta Diabetol Lat 1989;26(4):315-320. |
| 34. |
Ramachandran A, Susheela L, Mohan V, Kuzhali DA, Viswanathan M. Rapid
improvement in insulin binding to erythrocyte insulin receptors in
non-insulin-dependent diabetes mellitus during therapy. Acta Diabetol
Lat 1988;25(3):205-214. [Medline] [CrossRef] |
| 35. |
Das PK,
Bray GM, Aguayo AJ, Rasminsky M. Diminished ouabain-sensitive,
sodium-potassium ATPase activity in sciatic nerves of rats with
streptozotocin-induced diabetes. Exp Neurol 1976;53(1):285-288. [Medline] [CrossRef] |
| 36. |
Gnanaprakasam MS, Srivastava LM. Effect of starvation, alloxan diabetes
and adrenalectomy on Na+ K+-ATPase of the mucosa of the small intestine
of rat. Biochem Exp Biol 1978;14(3):257-262. [Medline] |
| 37. |
Greene
DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and
sodium-potassium-ATPase in the pathogenesis of diabetic complications. N
Engl J Med 1987;316(10):599-606. [Medline] |
| 38. |
Sima AA, Sugimoto K. Experimental diabetic neuropathy: an update.
Diabetologia 1999;42(7):773-788. [Medline] [CrossRef] |
| 39. |
Djemli-Shipkolye A, Raccah D, Pieroni G, Vague P, Coste TC, Gerbi A.
Differential effect of omega3 PUFA supplementations on Na,K-ATPase and
Mg-ATPase activities: possible role of the membrane omega6/omega3 ratio.
J Membr Biol 2003;191(1):37-47. [Medline] [CrossRef] |
| 40. |
Hundal
HS, Marette A, Mitsumoto Y, Ramlal T, Blostein R, Klip A. Insulin
induces translocation of the alpha 2 and beta 1 subunits of the
Na+/K(+)-ATPase from intracellular compartments to the plasma membrane
in mammalian skeletal muscle. J Biol Chem 1992;267(8):5040-5043. [Medline] |
| 41. |
Vague P,
Coste TC, Jannot MF, Raccah D, Tsimaratos M. C-peptide, Na+,K(+)-ATPase,
and diabetes. Exp Diabesity Res 2004;5(1):37-50. [Medline] [CrossRef] |
| 42. |
Djemli-Shipkolye A, Gallice P, Coste T, Jannot MF, Tsimaratos M, Raccah
D, Vague P. The effects ex vivo and in vitro of insulin and C-peptide on
Na/K adenosine triphosphatase activity in red blood cell membranes of
type 1 diabetic patients. Metabolism 2000;49(7):868-872. [Medline] [CrossRef] |
| 43. |
Mimura M,
Makino H, Kanatsuka A, Asai T, Yoshida S. Reduction of erythrocyte
(Na(+)-K+)ATPase activity in type 2 (non-insulin-dependent) diabetic
patients with microalbuminuria. Horm Metab Res 1994;26(1):33-38. [Medline] [CrossRef] |
| 44. |
Nagamatsu
S, Inoue N, Murakawa S, Matsui H. Evaluation of sodium and potassium
pump activity and number in diabetic erythrocytes. Acta Endocrinol (Copenh)
1986;111(1):69-74. [Medline] |
| 45. |
Suhail M, Rizvi SI. Red cell membrane (Na+ +K+)-ATPase in diabetes
mellitus. Biochem Biophys Res Commun 1987;146(1):179-186. [Medline] [CrossRef] |
| 46. |
Baldini P, Incerpi S, Lambert-Gardini S, Spinedi A, Luly P. Membrane
lipid alterations and Na+-pumping activity in erythrocytes from IDDM and
NIDDM subjects. Diabetes 1989;38(7):825-831. [Medline] [CrossRef] |
| 47. |
Finotti P, Palatini P. Reduction of erythrocyte (Na+-K+)ATPase activity
in type 1 (insulin-dependent) diabetic subjects and its activation by
homologous plasma. Diabetologia 1986;29(9):623-628. [Medline] [CrossRef] |
| 48. |
Rabini RA, Fumelli P, Staffolani R, Mazzanti L, Pugnaloni A, Biagini G,
Faloia E, et al. Effects of diabetes mellitus on structural and
functional properties of erythrocyte membranes. Membr Biochem
1993;10(2):71-79. [Medline] [CrossRef] |
| 49. |
Issautier
T, Kovacic H, Gallice P, Raccah D, Vague P, Crevat A. Modulation defect
of sodium pump evidenced in diabetic patients by a microcalorimetric
study. Clin Chim Acta 1994;228(2):161-170. [Medline] [CrossRef] |
| 50. |
Forst T, Kunt T. Effects of C-peptide on microvascular blood flow and
blood hemorheology. Exp Diabesity Res 2004;5(1):51-64. [Medline] [CrossRef] |
| 51. |
Dostanic-Larson
I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac
glycoside binding site of Na,K-ATPase plays a role in blood pressure
regulation. Proc Natl Acad Sci U S A 2005;102(44):15845-15850. [Medline] [CrossRef] |
| 52. |
Ferrari
P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain
antagonist that corrects renal and vascular Na+-K+- ATPase alterations
in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr
Comp Physiol 2006;290(3):R529-535. [Medline] [CrossRef] |
| 53. |
Weidmann P, Ferrari P. Central role of sodium in hypertension in
diabetic subjects. Diabetes Care 1991;14(3):220-232. [Medline] [CrossRef] |
| 54. |
Tirupattur PR, Ram JL, Standley PR, Sowers JR. Regulation of Na+,K(+)-ATPase
gene expression by insulin in vascular smooth muscle cells. Am J
Hypertens 1993;6(7 Pt 1):626-629. [Medline] |
| 55. |
Aperia A. Regulation of sodium/potassium ATPase activity: impact on salt
balance and vascular contractility. Curr Hypertens Rep
2001;3(2):165-171. [Medline] [CrossRef] |
| 56. |
Wang X,
Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal
tubular salt transport in hypertension: an update. Curr Opin Nephrol
Hypertens 2009;18(5):412-420. [Medline] [CrossRef] |
| 57. |
Garay R, Adragna N, Canessa M, Tosteson D. Outward sodium and potassium
cotransport in human red cells. J Membr Biol 1981;62(3):169-174. [Medline] [CrossRef] |
| 58. |
Zidek W, Losse H, Schmidt W, Vetter H. Potassium load in spontaneously
hypertensive rats. Effects on blood pressure, renin-angiotensin,
aldosterone, and intracellular electrolytes. Res Exp Med (Berl)
1983;183(2):147-152. [Medline] [CrossRef] |
| 59. |
Akimova
OA, Bagrov AY, Lopina OD, Kamernitsky AV, Tremblay J, Hamet P, Orlov SN.
Cardiotonic steroids differentially affect intracellular Na+ and [Na+]i/[K+]i-independent
signaling in C7-MDCK cells. J Biol Chem 2005;280(1):832-839. [Medline] |
| 60. |
Orlov SN, Hamet P. The death of cardiotonic steroid-treated cells:
evidence of Na+i,K+i-independent H+i-sensitive signalling. Acta Physiol
(Oxf) 2006;187(1-2):231-240. [Medline] [CrossRef] |
| 61. |
Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac
glycosides: their roles in hypertension, salt metabolism, and cell
growth. Am J Physiol Cell Physiol 2007;293(2):C509-536. [Medline] [CrossRef] |
| 62. |
Schwinger
RH, Bundgaard H, Muller-Ehmsen J, Kjeldsen K. The Na, K-ATPase in the
failing human heart. Cardiovasc Res 2003;57(4):913-920. [Medline] [CrossRef] |
| 63. |
Kjeldsen
K. Myocardial Na,K-ATPase: Clinical aspects. Exp Clin Cardiol
2003;8(3):131-133. [Medline] |
| 64. |
Muller-Ehmsen
J, McDonough AA, Farley RA, Schwinger RH. Sodium pump isoform expression
in heart failure: implication for treatment. Basic Res Cardiol 2002;97
Suppl 1:I25-30. [Medline] |
| 65. |
Stefanon
I, Cade JR, Fernandes AA, Ribeiro Junior RF, Targueta GP, Mill JG,
Vassallo DV. Ventricular performance and Na+-K+ ATPase activity are
reduced early and late after myocardial infarction in rats. Braz J Med
Biol Res 2009;42(10):902-911. [Medline] [CrossRef] |
| 66. |
Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The
alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and
functionally interacts and co-localizes with the Na/Ca exchanger in
heart. J Biol Chem 2004;279(52):54053-54061.
|
| 67. |
Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na(+)
concentration is elevated in heart failure but Na/K pump function is
unchanged. Circulation 2002;105(21):2543-2548. |
| 68. |
Han F,
Tucker AL, Lingrel JB, Despa S, Bers DM. Extracellular potassium
dependence of the Na+-K+-ATPase in cardiac myocytes: isoform specificity
and effect of phospholemman. Am J Physiol Cell Physiol
2009;297(3):C699-705. [Medline] [CrossRef] |
| 69. |
McDonough
AA, Velotta JB, Schwinger RH, Philipson KD, Farley RA. The cardiac
sodium pump: structure and function. Basic Res Cardiol 2002;97 Suppl
1:I19-24. [Medline] |
| 70. |
Vlkovicova J, Javorkova V, Mezesova L, Pechanova O, Vrbjar N. Regulatory
role of nitric oxide on the cardiac Na, K-ATPase in hypertension.
Physiol Res 2008;57 Suppl 2:S15-22. [Medline] |
| 71. |
Petrushanko IY, Bogdanov NB, Lapina N, Boldyrev AA, Gassmann M,
Bogdanova AY. Oxygen-induced Regulation of Na/K ATPase in cerebellar
granule cells. J Gen Physiol 2007;130(4):389-398. [Medline] [CrossRef] |
| 72. |
Newton
LD, Krishnakumar S, Menon AG, Kastelic JP, van der Hoorn FA, Thundathil
JC. Na+/K+ATPase regulates sperm capacitation through a mechanism
involving kinases and redistribution of its testis-specific isoform. Mol
Reprod Dev 2009;77(2):136-148. [Medline] |
| 73. |
Thundathil JC, Anzar M, Buhr MM. Na+/K+ATPase as a signaling molecule
during bovine sperm capacitation. Biol Reprod 2006;75(3):308-317.
[Medline] [CrossRef] |
| 74. |
Lang F.
Mechanisms and significance of cell volume regulation. J Am Coll Nutr
2007;26(5 Suppl):613S-623S. [Medline] |
| 75. |
Panayiotidis MI, Bortner CD, Cidlowski JA. On the mechanism of ionic
regulation of apoptosis: would the Na+/K+-ATPase please stand up? Acta
Physiol (Oxf) 2006;187(1-2):205-215.
[Medline] [CrossRef] |
| 76. |
Testa I,
Rabini RA, Corvetta A, Danieli G. Decreased NA+, K+-ATPase activity in
erythrocyte membrane from rheumatoid arthritis patients. Scand J
Rheumatol 1987;16(4):301-305.
[Medline] [CrossRef] |
| 77. |
Kiziltunc A, Cogalgil S, Ugur M, Avci B, Akcay F. Sialic acid,
transketolase and Na+, K+, ATPase in patients with rheumatoid arthritis.
Clin Chem Lab Med 1998;36(5):289-293. [Medline] [CrossRef] |
| 78. |
Hsieh CC,
Hwang TL, Chen HM, Chen MF, Sun YF, Lau YT. Sepsis correlated with
increased erythrocyte Na+ content and Na+ - K+ pump activity. J Biomed
Sci 2003;10(4):389-395. [Medline] |
| 79. |
Tsakadze
LG, Kometiani ZP. [The regulation of the Na, K-ATPase system by
neurotransmitters]. Nauchnye Doki Vyss Shkoly Biol Nauki 1990;6:16-26.
[Medline] |
| 80. |
Kurup RK, Kurup PA. Schizoid neurochemical pathology-induced membrane
Na(+)-K+ ATPase inhibition in relation to neurological disorders. Int J
Neurosci 2003;113(12):1705-1717. [Medline] [CrossRef] |
| 81. |
Harrington MG, Fonteh AN, Arakaki X, Cowan RP, Ecke LE, Foster H, Huhmer
AF, et al. Capillary Endothelial Na(+), K(+), ATPase Transporter
Homeostasis and a New Theory for Migraine Pathophysiology. Headache
2009. [Medline] |
| 82. |
Koenderink JB, Zifarelli G, Qiu LY, Schwarz W, De Pont JJ, Bamberg E,
Friedrich T. Na,K-ATPase mutations in familial hemiplegic migraine lead
to functional inactivation. Biochim Biophys Acta 2005;1669(1):61-68.
[Medline] [CrossRef] |
| 83. |
Delamere
NA, Tamiya S. Expression, regulation and function of Na,K-ATPase in the
lens. Prog Retin Eye Res 2004;23(6):593-615. [Medline] [CrossRef] |
| 84. |
Sznajder
JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of
Na(+)-K(+)-ATPase. J Appl Physiol 2002;93(5):1860-1866. [Medline] |
| 85. |
Matthay
MA, Clerici C, Saumon G. Invited review: Active fluid clearance from the
distal air spaces of the lung. J Appl Physiol 2002;93(4):1533-1541.
[Medline] |
| 86. |
Kaminsky
P, Klein M, Duc M. [Hypothyroid myopathy. Physiopathological approach].
Ann Endocrinol (Paris) 1992;53(4):125-132. [Medline] |
| 87. |
Bhargava
M, Lei J, Mariash CN, Ingbar DH. Thyroid hormone rapidly stimulates
alveolar Na,K-ATPase by activation of phosphatidylinositol 3-kinase.
Curr Opin Endocrinol Diabetes Obes 2007;14(5):416-420.
[Medline] |
| 88. |
Phakdeekitcharoen B, Phudhichareonrat S, Pookarnjanamorakot C,
Kijkunasathian C, Tubtong N, Kittikanokrat W, Radinahamed P. Thyroid
hormone increases mRNA and protein expression of Na+-K+-ATPase alpha2
and beta1 subunits in human skeletal muscles. J Clin Endocrinol Metab
2007;92(1):353-358. [Medline] [CrossRef] |
| 89. |
Feraille
E, Mordasini D, Gonin S, Deschenes G, Vinciguerra M, Doucet A,
Vandewalle A, et al. Mechanism of control of Na,K-ATPase in principal
cells of the mammalian collecting duct. Ann N Y Acad Sci
2003;986:570-578. [Medline] |
| 90. |
Vinciguerra M, Mordasini D, Vandewalle A, Feraille E. Hormonal and
nonhormonal mechanisms of regulation of the NA,K-pump in collecting duct
principal cells. Semin Nephrol 2005;25(5):312-321. [Medline] [CrossRef] |
| 91. |
Kamenicky P, Viengchareun S, Blanchard A, Meduri G, Zizzari P,
Imbert-Teboul M, Doucet A, et al. Epithelial sodium channel is a key
mediator of growth hormone-induced sodium retention in acromegaly.
Endocrinology 2008;149(7):3294-3305. [Medline] [CrossRef] |
| 92. |
Suhail M, Faizul Suhail M, Khan H. Role of vitamins C and e in
regulating antioxidant and pro-oxidant markers in preeclampsia. J Clin
Biochem Nutr 2008;43(3):210-220. [Medline] [CrossRef] |
| 93. |
Graves
SW. Sodium regulation, sodium pump function and sodium pump inhibitors
in uncomplicated pregnancy and preeclampsia. Front Biosci
2007;12:2438-2446. [Medline] |
| 94. |
Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D,
Rajasekaran AK. Na,K-ATPase inhibition alters tight junction structure
and permeability in human retinal pigment epithelial cells. Am J Physiol
Cell Physiol 2003;284(6):C1497-1507. [Medline] |
| 95. |
Blok LJ,
Chang GT, Steenbeek-Slotboom M, van Weerden WM, Swarts HG, De Pont JJ,
van Steenbrugge GJ, et al. Regulation of expression of Na+,K+-ATPase in
androgen-dependent and androgen-independent prostate cancer. Br J Cancer
1999;81(1):28-36. [Medline] [CrossRef] |
| 96. |
Espineda CE, Chang JH, Twiss J, Rajasekaran SA, Rajasekaran AK.
Repression of Na,K-ATPase beta1-subunit by the transcription factor
snail in carcinoma. Mol Biol Cell 2004;15(3):1364-1373. [Medline] [CrossRef] |
| 97. |
Barwe SP, Anilkumar G, Moon SY, Zheng Y, Whitelegge JP, Rajasekaran SA,
Rajasekaran AK. Novel role for Na,K-ATPase in phosphatidylinositol
3-kinase signaling and suppression of cell motility. Mol Biol Cell
2005;16(3):1082-1094. [Medline] [CrossRef] |
| 98. |
Seligson DB, Rajasekaran SA, Yu H, Liu X, Eeva M, Tze S, Ball W, Jr., et
al. Na,K-adenosine triphosphatase alpha1-subunit predicts survival of
renal clear cell carcinoma. J Urol 2008;179(1):338-345. [Medline] [CrossRef] |
| 99. |
Mijatovic T, Ingrassia L, Facchini V, Kiss R. Na+/K+-ATPase
alpha subunits as new targets in anticancer therapy. Expert Opin Ther
Targets 2008;12(11):1403-1417. |